Silver iodate thermochromic material collaboratively constructed on basis of ionic bonds and coordination bonds and preparation method of silver iodate thermochromic material
A technology of thermochromic materials and iodine silver salts, applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of inconspicuous discoloration of thermochromic materials, achieve easy observation, reduce operation time, fast response effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
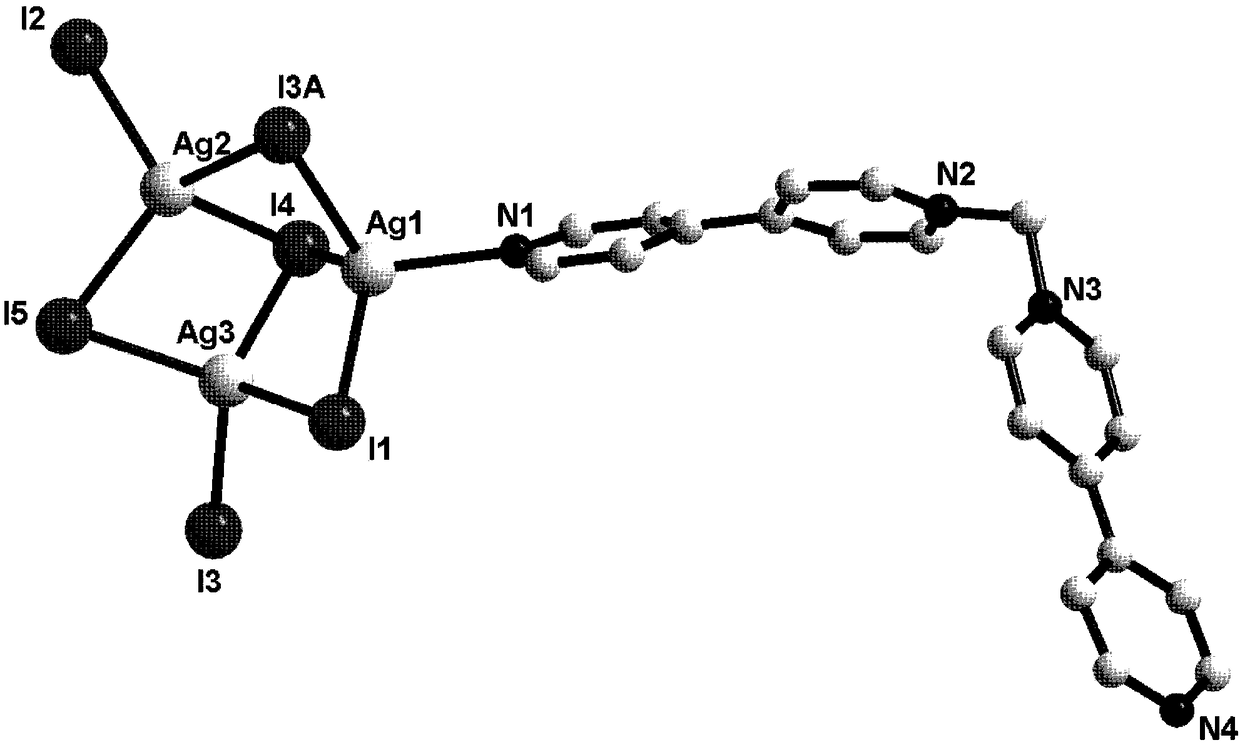
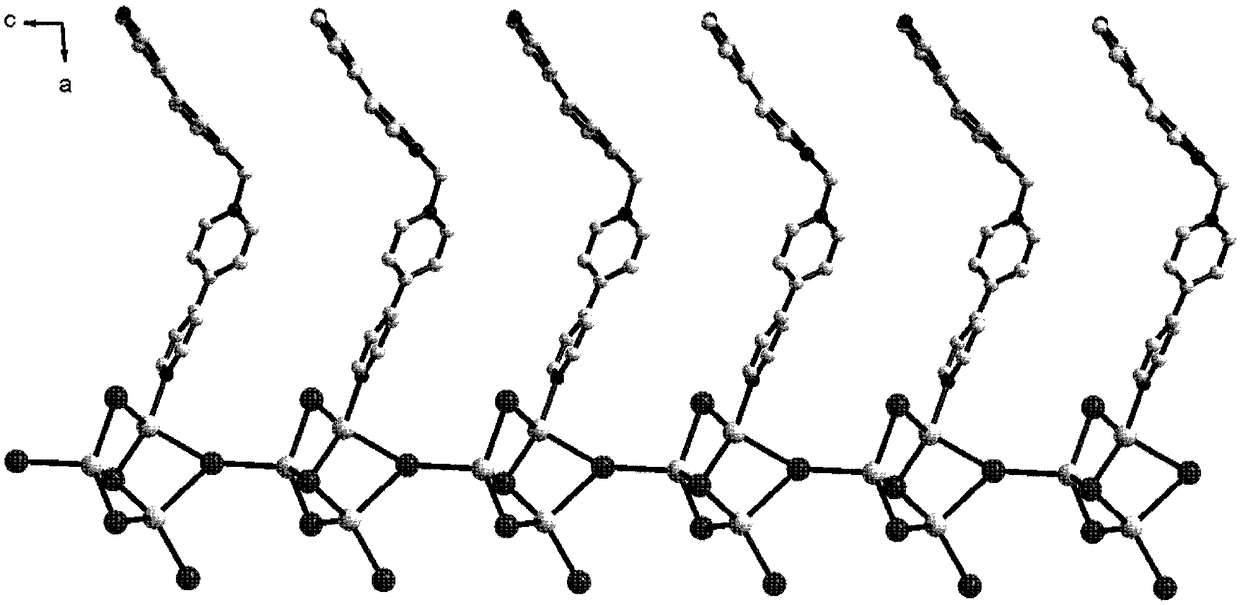
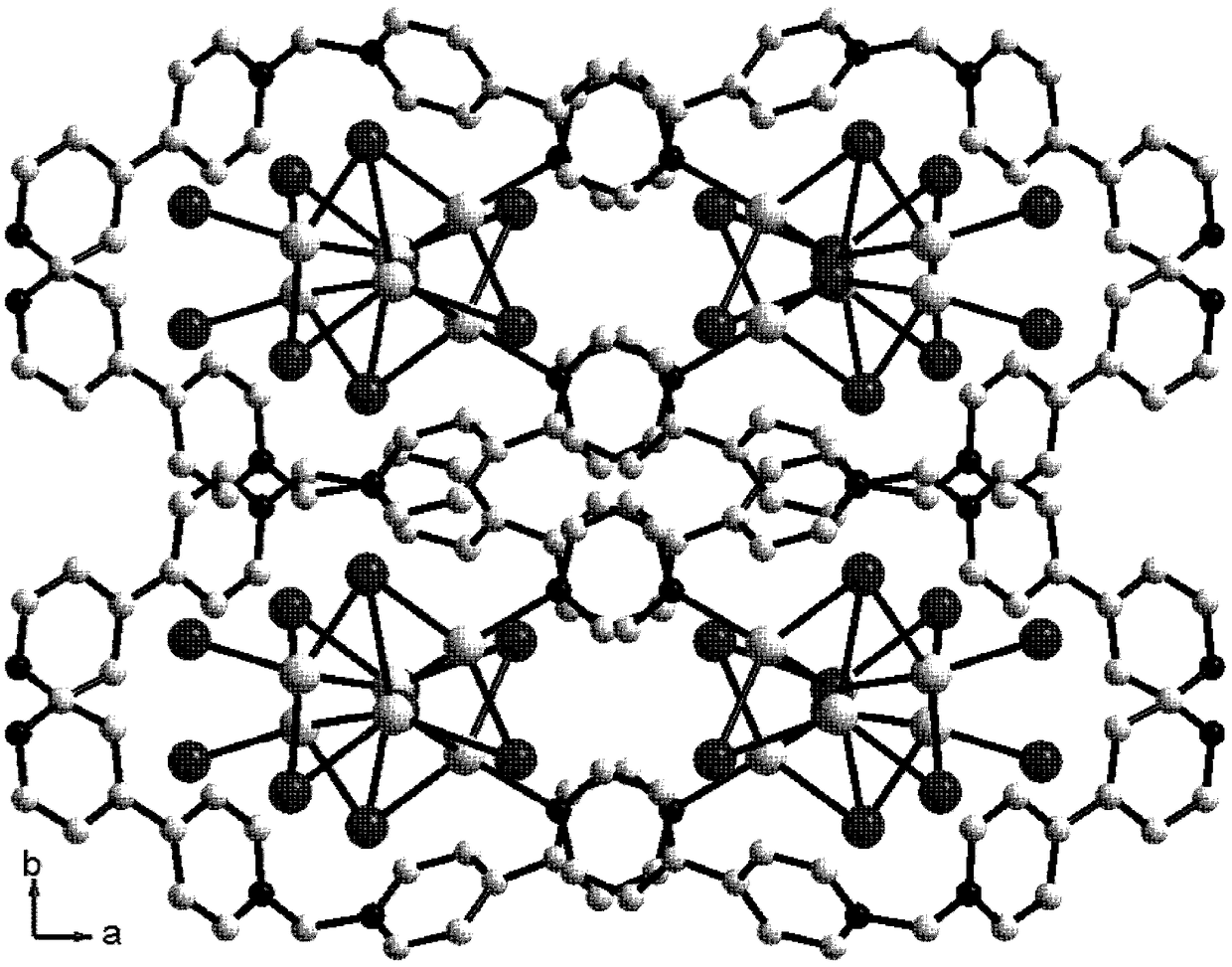
[0034] 1,1′-methylene-bis(4,4′-bipyridine) dibromide (MBP·Br 2) (0.1mmol, 0.048g), a mass fraction of 45% HI (1.0mmol, 0.284g) solution and AgI (0.3mmol, 0.071g) were added to the 7ml DMF solution in turn, and 10 drops of water were added dropwise with a rubber dropper and Stir to obtain a deep red solution, place the volatile solvent at room temperature to obtain a deep red diamond-shaped flaky crystal, wash with ethanol for 2-3 times and dry to obtain [(MBP)(Ag 3 I 5 )] n . Yield: 70.1% (based on Ag). Elemental analysis: C 21 h 18 N 4 Ag 3 I 5 : C, 19.63; H, 1.41; N, 4.36. Found: C, 19.58; H, 1.50, N, 4.31.
Embodiment 2
[0036] 1,1'-methylene-bis(4,4-bipyridyl) dibromide (MBP·Br 2 ) (0.1mmol, 0.048g), mass fraction of 45% HI (1.0mmol, 0.284g) solution and AgI (0.3mmol, 0.071g), were sequentially added to 3ml DMF solution and dripped 5 drops of water with a rubber dropper to obtain Dark red solution, place volatile solvent at room temperature to obtain deep red diamond-shaped flaky crystals, wash with ethanol 2-3 times and dry to obtain [(MBP)(Ag 3 I 5 )] n . Yield: 43.2% (based on Ag).
Embodiment 3
[0038] 1,1'-methylene-bis(4,4-bipyridyl) dibromide (MBP·Br 2 ) (0.1mmol, 0.048g), a mass fraction of 45% HI (1.0mmol, 0.284g) solution and AgI (0.3mmol, 0.071g) were added to the 5ml DMF solution in turn, and 10 drops of water were added dropwise with a rubber dropper. Stir to obtain a deep red solution, place the volatile solvent at room temperature to obtain a deep red diamond-shaped flaky crystal, wash with ethanol for 2-3 times and dry to obtain [(MBP)(Ag 3 I 5 )] n . Yield: 55.5% (based on Ag).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com