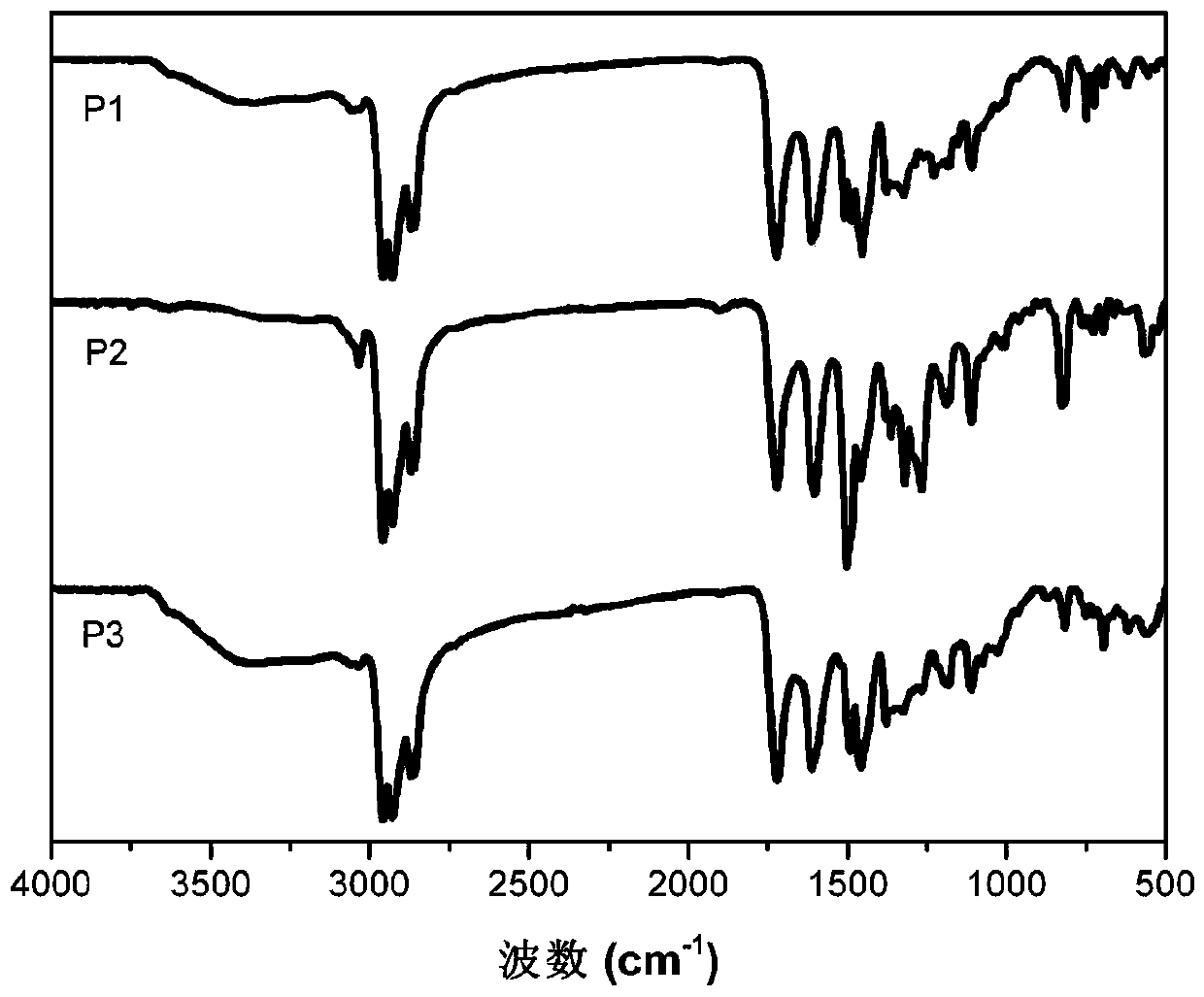
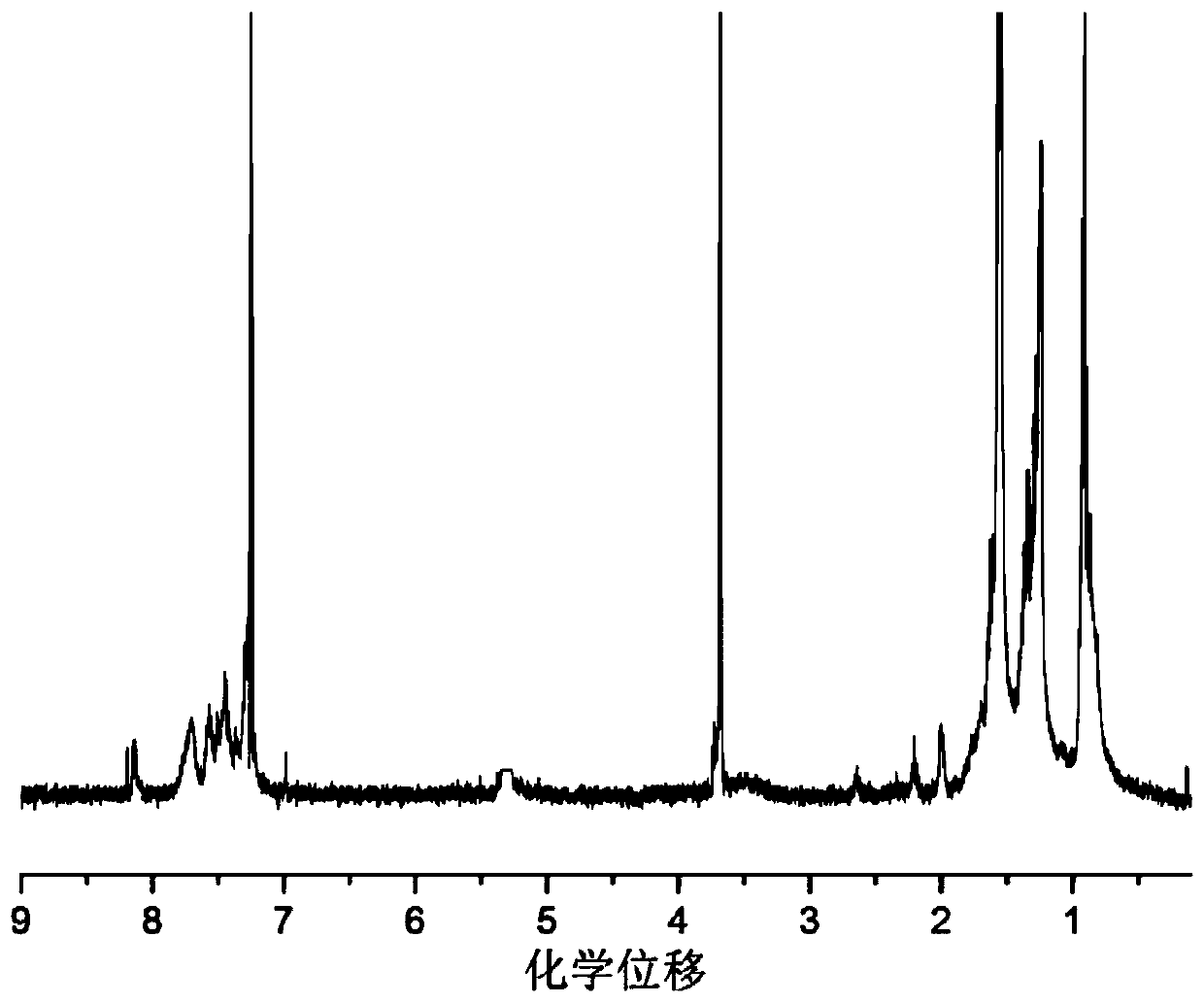
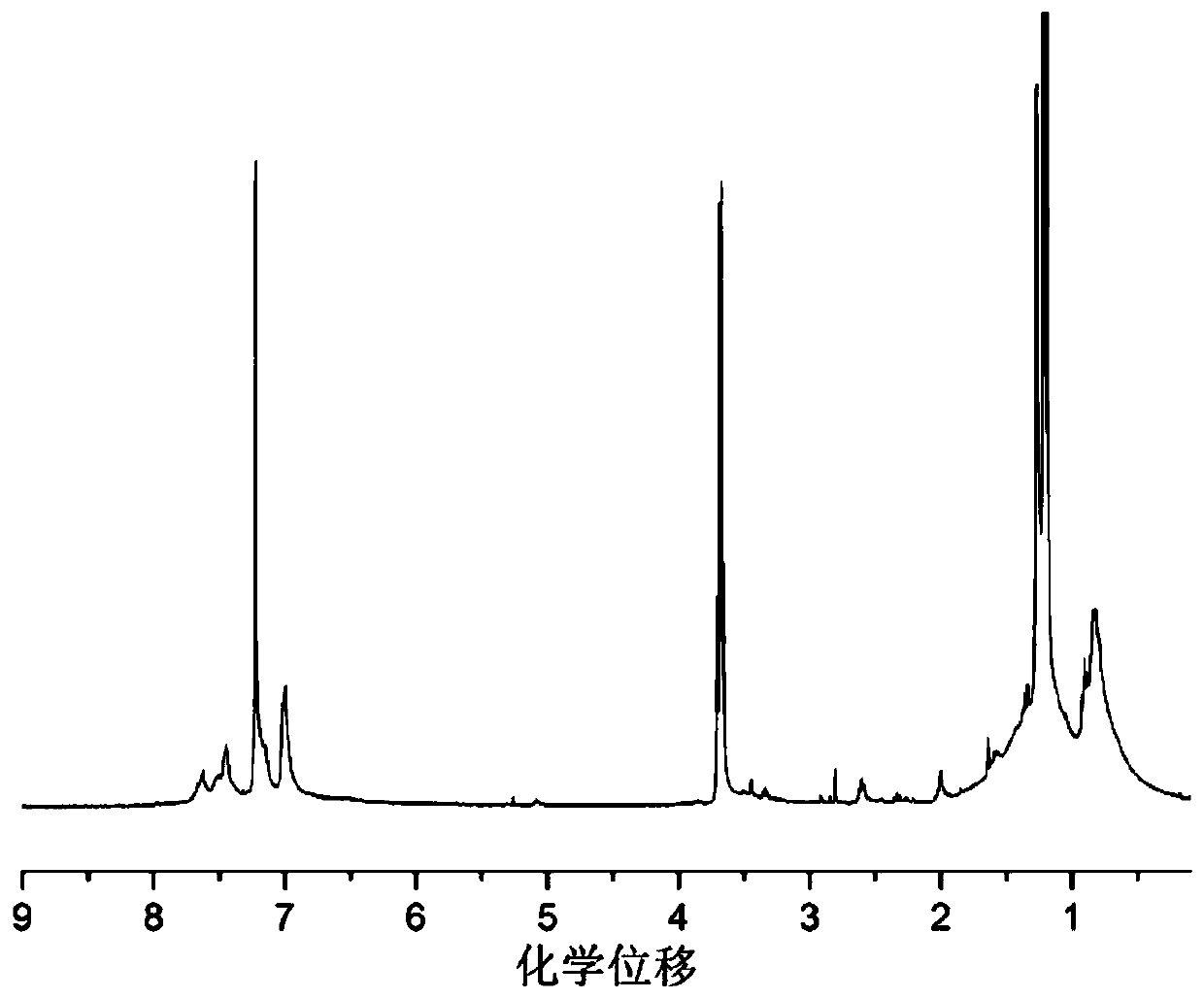
Triphenylamine isoindigo polymer containing biphenyl structure as well as preparation method and application thereof
A technology of biphenyl structure and triphenylamine, applied in the field of triphenylamine-like isoindigo polymers, can solve the problems of low solubility and narrow application range of isoindigo, and achieve obvious discoloration, excellent electrochromic performance, and high hole mobility. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0057] Specific embodiment 1: The triphenylamine-based isoindigo polymer containing biphenyl structure in this embodiment is carbazolyltriphenylamine-based isoindigo polymer containing biphenyl structure or bis-4-tert-butyltriphenylamine containing biphenyl structure isoindigo polymer or triphenylaminoisoindigo polymer containing biphenyl structure;
[0058] Wherein the structural formula of the carbazolyl triphenylamino-isoindigo polymer containing biphenyl structure is:
[0059] In the formula, n is a positive integer;
[0060] The structural formula of two 4-tert-butyltriphenylamino-isoindigo polymers containing biphenyl structure is:
[0061] In the formula, n is a positive integer.
[0062] The structural formula of the triphenylamino-isoindigo polymer containing biphenyl structure is:
[0063] In the formula, n is a positive integer.
[0064] This embodiment has the following beneficial effects:
[0065] 1. The isoindigo unit has two strong electron-withdrawin...
specific Embodiment approach 2
[0070] Specific embodiment two: the method for the triphenylamine class isoindigo polymer containing biphenyl structure of this embodiment is carried out according to the following steps:
[0071] 1. Preparation of isoindigo derivatives:
[0072] ①. Add 6-bromoisoindole and anhydrous potassium carbonate to anhydrous N,N-dimethylformamide to form a suspension, and then inject 1-bromo-2-ethylhexyl into the suspension under a nitrogen atmosphere alkane to obtain a mixture; the mixture was stirred at 99-100°C for 14-15 hours and then poured into water, using CH 2 Cl 2 Extract as the organic phase to obtain the extracted organic phase, then add anhydrous MgSO 4 Drying is carried out, and the dried organic phase is subjected to vacuum distillation to obtain a solid product, and the solid product is separated by a chromatographic column to obtain compound M1;
[0073] The ratio of the amount of substance of anhydrous potassium carbonate and 6-bromoisoindole described in step 1. is...
specific Embodiment approach 3
[0088] Specific embodiment three: the difference between this embodiment and specific embodiment two is: the preparation method of the degassed tetrahydrofuran described in step ② is: vacuumize the three-neck round bottom flask filled with tetrahydrofuran with a vacuum pump, and then pass it into Nitrogen: Repeat vacuuming and feeding nitrogen 3 to 4 times. Others are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com