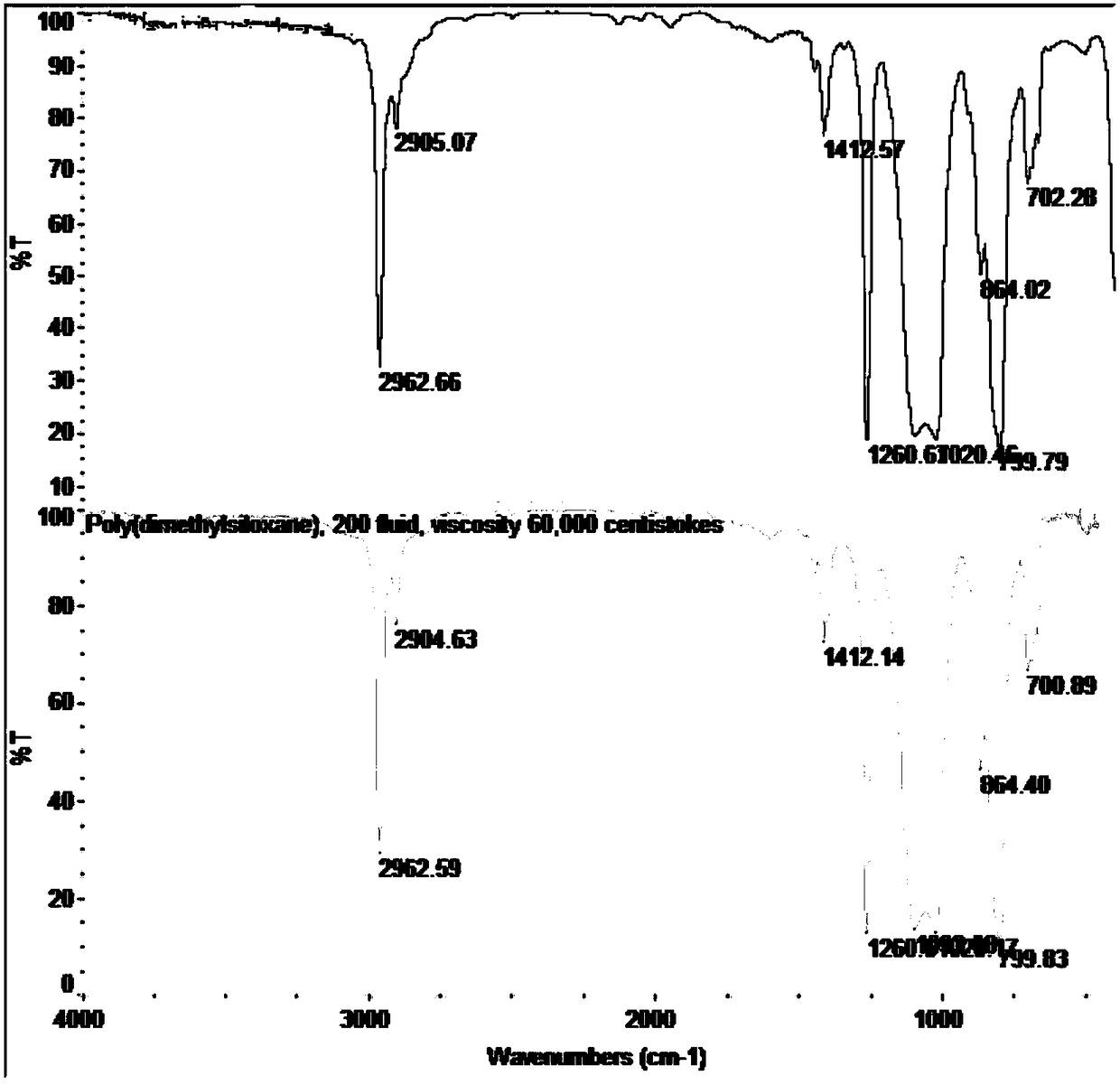
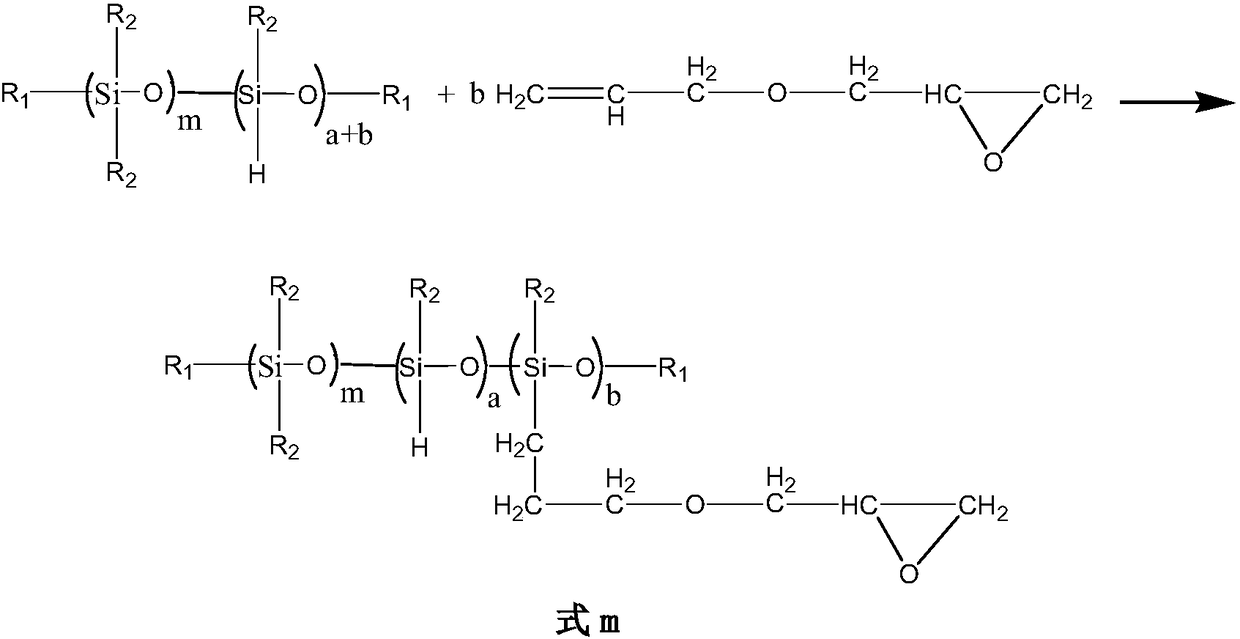
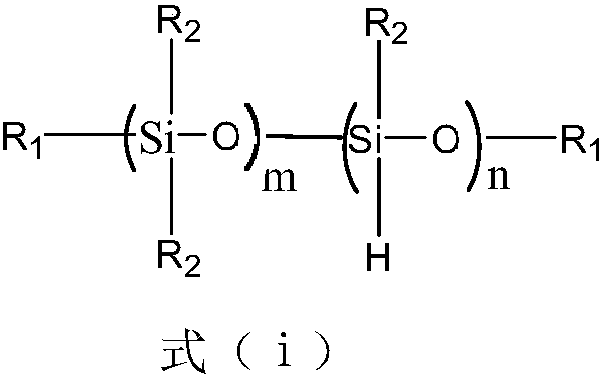Unsaturated epoxidized polysiloxane, preparation method and applications thereof
A technology for epoxidized polysiloxane and hydrogen polysiloxane, which is applied in the field of preparation of epoxidized silicone oil modification, can solve the problem of large amount of epoxidized silicone oil, insufficient conversion rate of silicon-hydrogen, and low-hydrogen silicone oil conversion rate of less than 85%. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Add 100g of anhydrous vinyl polydimethylhydrogensiloxane (viscosity of 15000mPa·s, vinyl content of 0.56mol%, hydrogen content of 0.02%) into a dried 500mL four-necked flask, and dry over molecular sieves 100mL of anhydrous toluene, high-purity nitrogen was introduced to drive away the air in the bottle and protect the reaction system, 2.5mL of 0.1wt% chloroplatinic acid toluene solution was added dropwise, the temperature was controlled at 50 °C, and 2.9g of allyl glycidol Add the ether into the separatory funnel and drop it into the reaction solution within 1 h, stir and react at this temperature for 5.5 h, after the reaction is over, add 100 mL of ion-free water to wash the reaction solution for 15 min, then separate the water phase at room temperature, Then vacuum removed low boilers, solvent and unreacted allyl glycidyl ether at about 90° C. to obtain light yellow transparent liquid, and the residual active hydrogen content in the product was measured to be 3.0 mmol...
Embodiment 2
[0086] Add 100 g of anhydrous vinyl polymethylvinyl hydrogen-containing siloxane (viscosity of 18000 mPa·s, vinyl content of 0.98 mol%, hydrogen content of 0.04 mol%) into a dried 500 mL four-necked flask, molecular sieve Dried anhydrous toluene 100mL, control the temperature at about 50°C. Add 4.4mL of 0.1wt% chloroplatinic acid toluene solution dropwise, feed high-purity nitrogen to drive away the air in the bottle and protect the reaction system, control the temperature at 65°C, and then Add 5.3g of allyl glycidyl ether into the separatory funnel and drop it into the reaction solution within 2h, stir and react at this temperature for 8h, after the reaction is over, add 100mL of ion-free water to wash the reaction solution for 15min, and then in The water phase was separated at room temperature, and then the low boilers, solvent and unreacted allyl glycidyl ether were removed in vacuum at about 120°C to obtain a light yellow transparent liquid, and the residual active hydroge...
Embodiment 3
[0089] Add 100g of vinyl-terminated polydiethylhydrosiloxane (viscosity of 58000mPa·s, vinyl content of 0.85mol%, hydrogen content of 0.08%), molecular sieve-dried non- Water toluene 100mL, feed high-purity nitrogen to drive away the air in the bottle and protect the reaction system, add dropwise 0.1wt% chloroplatinic acid toluene solution 4.2mL, control the temperature at 85 °C, and then add 11g of 1,2-epoxy- Add 4-vinylcyclohexane into the separatory funnel and drop it into the reaction solution within 1.5h, stir and react at this temperature for 5.5h, after the reaction is over, add 100mL deionized water to wash the reaction solution for 15min, and then The water phase was separated at room temperature, and the low boilers, solvent and unreacted 1,2-epoxy-4-vinylcyclohexane were removed in vacuum at about 150°C to obtain a light yellow transparent liquid. As a result, the residual active hydrogen content in the pale yellow transparent liquid product was 12 mmol%.
[0090] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com