Pharmaceutical composition containing 18 types of amino acids and preparation method thereof
A composition, amino acid technology, applied in the field of medicine, can solve problems such as adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] The preparation of embodiment 1 compound amino acid injection 18AA
[0120] The components and dosages of Compound Amino Acid Injection 18AA are shown in the table below:
[0121] Tyrosine
0.50g
0.25g
10.00g
Lysine acetate
7.00g
7.50g
3.00g
5.00g
3.00g
0.25g
1.50g
7.50g
2.50g
2.50g
1.00g
2.00g
2.50g
5.00g
0.25g
0.1mol / L acetic acid solution
Appropriate amount
Water for Injection
Appropriate amount
[0122] The total amount is 1000ml.
[0123] Preparation:
[0124] (1) Weigh each raw material and auxiliary material according to the prescription;
[0125] (2) Take 70% of the total amount of water for injection, keep the water temperature a...
Embodiment 2
[0131] The preparation of embodiment 2 compound amino acid injection 18AA
[0132] The components and dosages of Compound Amino Acid Injection 18AA are shown in the table below:
[0133] Tyrosine
0.475g
0.230g
9.200g
Lysine acetate
6.440g
6.900g
2.760g
4.750g
2.760g
0.230g
1.380g
6.900g
2.300g
2.300g
0.920g
1.840g
2.300g
4.600g
0.230g
0.1mol / L sodium acetate solution
Appropriate amount
Water for Injection
Appropriate amount
[0134] The total amount is 1000ml.
[0135] Preparation:
[0136] (1) Weigh each raw material and auxiliary material according to the prescription;
[0137] (2) Take 70% of the total amount of water for injection, keep the...
Embodiment 3
[0143] The preparation of embodiment 3 compound amino acid injection 18AA
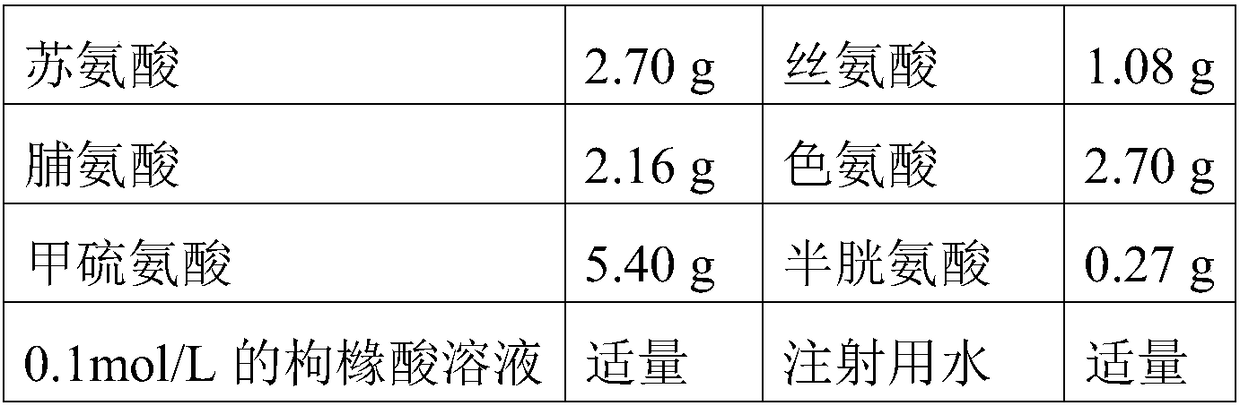
[0144] The components and dosages of Compound Amino Acid Injection 18AA are shown in the table below:
[0145]
[0146]
[0147] The total amount is 1000ml.
[0148] Preparation:
[0149] (1) Weigh each raw material and auxiliary material according to the prescription;
[0150] (2) Take 70% of the total amount of water for injection, keep the water temperature at 70° C., carry out nitrogen replacement operations on the reactor for 3 times, and feed nitrogen into the reactor for heat preservation.
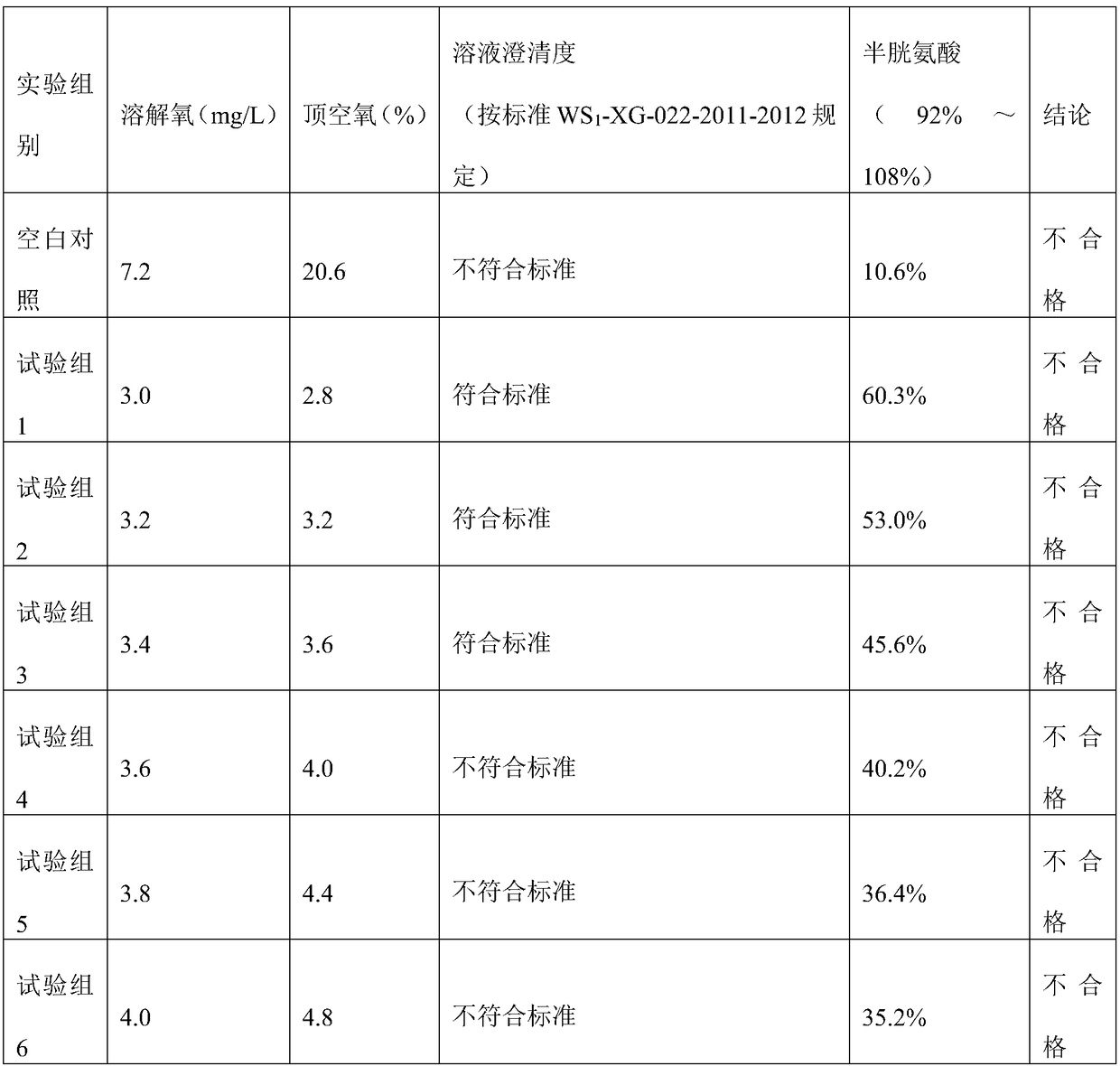
[0151] (3) Under the protection of nitrogen flow, monitor the dissolved oxygen below 0.5mg / L, add the prescribed amount of tyrosine, leucine, isoleucine, valine, aspartic acid, phenylalanine and Methionine. Stir until dissolved.
[0152] (4) Under the protection of nitrogen flow, cool down to 40°C, add the prescribed amount of threonine, proline, glutamic acid, lysine acetate, arginine, alanine, g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com