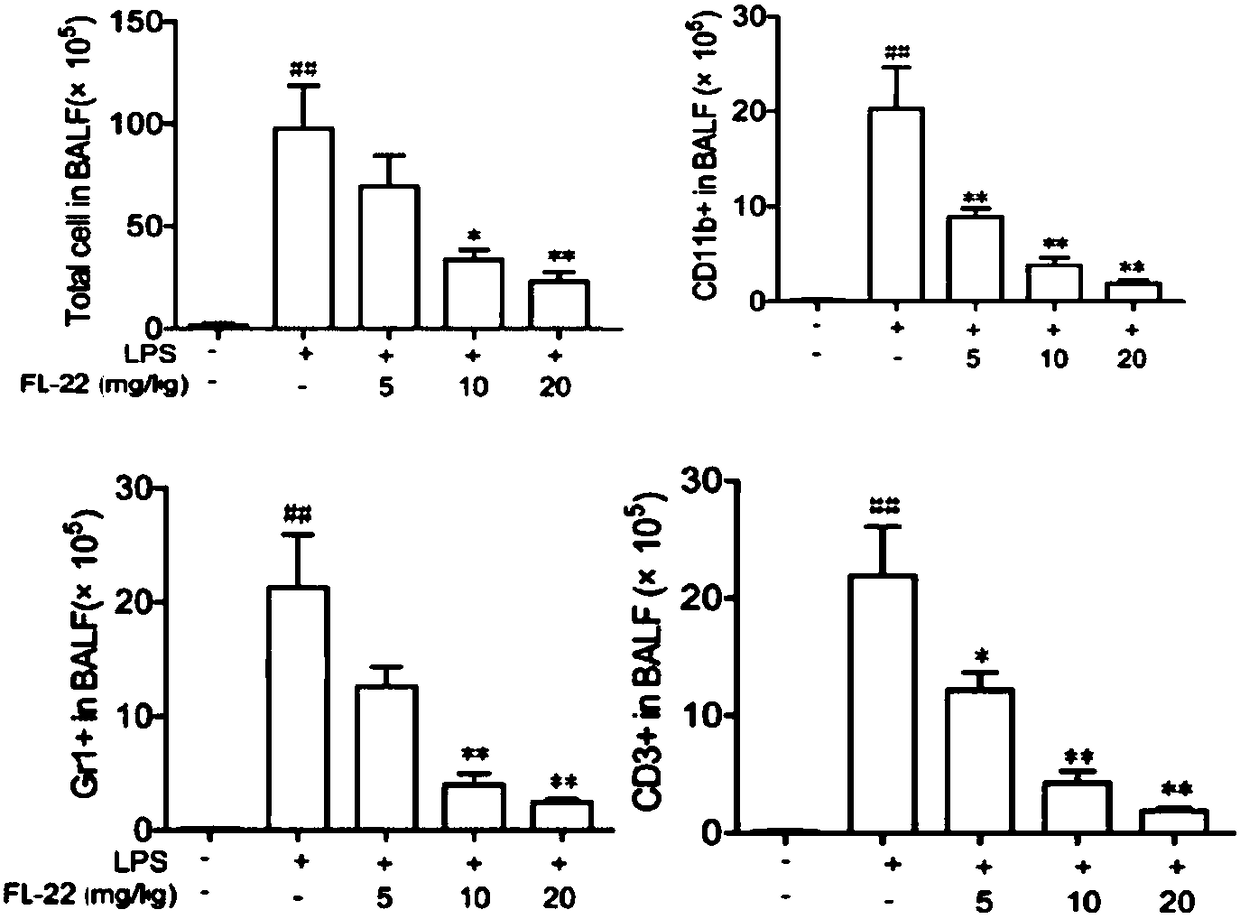
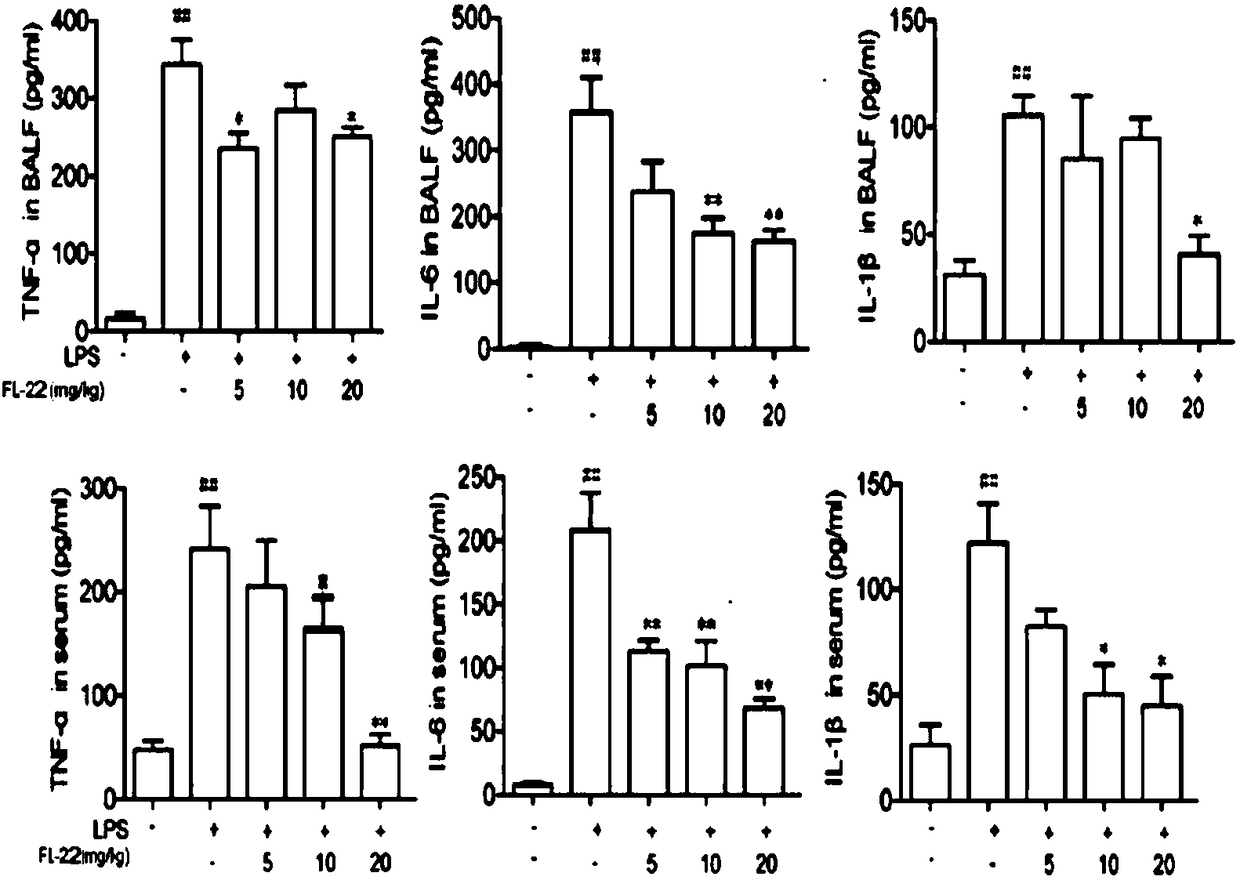
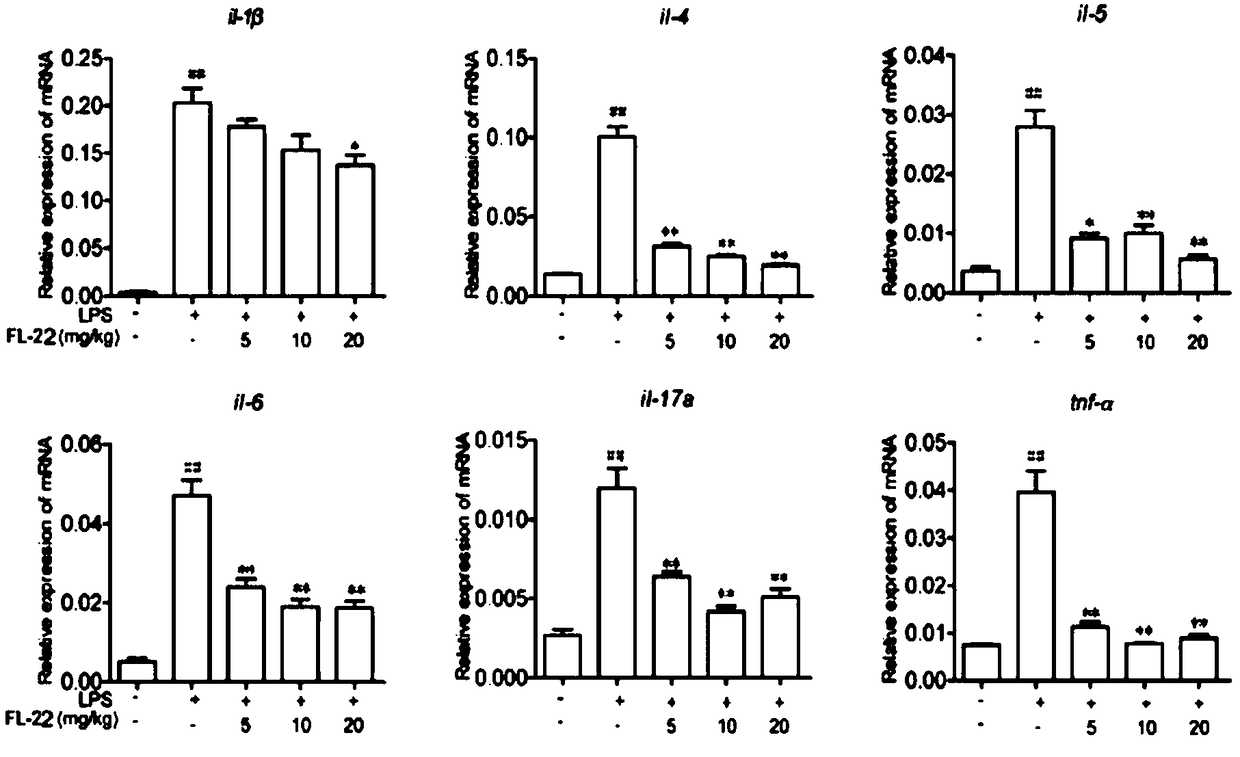
Application of ethyl potassium sulfate in preparation of drugs for preventing or treating inflammatory diseases
A technology for inflammatory diseases and potassium sulfate, which is applied in the direction of sulfate ester preparation, diseases, skin diseases, etc., can solve the problems of undiscovered medical uses of potassium ethyl sulfate, and achieve good anti-inflammatory effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: the preparation of potassium ethyl sulfate
[0044] Add a certain amount of ethanol to the reaction kettle, slowly add 6 times the amount of sulfuric acid ethanol solution dropwise under stirring, control the temperature of the reaction kettle at 38°C, and continue stirring for 24 hours for the esterification reaction. Add ethanol, stir well, adjust the pH to 7.0 with 10% KOH, add 95% ethanol to make the alcohol content reach more than 85%, recover ethanol under reduced pressure, and separate the aqueous solution through adsorption resin column chromatography, and use water: ethanol gradient elution, The eluate was collected and potassium ethyl sulfate was crystallized.
Embodiment 2
[0045] Embodiment 2: Potassium ethyl sulfate is to the influence of phorbol ester-induced mouse auricle swelling
[0046] experimental method
[0047]ICR mice, half male and half female, aged 6-8 weeks, weighing 18-22 g, were randomly divided into 6 groups (model group, five dose groups of potassium ethylsulfate). Use a 20 μL pipette to evenly apply 20 μL of TPA solution to the right ear of each mouse. Potassium ethyl sulfate was injected intravenously at a volume of 0.1 mL / 10 g 1 hour after modeling, and the dosages were 12.5, 25, 50, 100, and 200 mg / kg, respectively. After 6 hours of modeling, the mice were sacrificed by dislocation of the cervical vertebrae, and round ears were punched in the same part of the two auricles with a punch with a diameter of 8 mm, weighed with an electronic balance, and the swelling degree and swelling inhibition rate of the auricles were calculated according to the following formula. Degree of swelling (%)=(right ear mass−left ear mass) / left ...
Embodiment 3
[0056] Example 3: Effect of Potassium Ethyl Sulfate on Rat Paw Swelling Caused by Carrageenan
[0057] experimental method
[0058] Wistar rats, 200-220g, were randomly divided into 6 groups (normal group, model group, four dose groups of potassium ethyl sulfate). After chloral hydrate anesthesia, in addition to the normal group given the same amount of normal saline, the rats in other groups were injected subcutaneously with 0.1 mL of sterile 1% carrageenan normal saline suspension on the right hind paw to cause inflammation, and 0.5 hours after inflammation Different doses of potassium ethyl sulfate or normal saline were administered intravenously, and the administration volume was 1 mL / 100 g. The water volume method was used to measure the volume of the right hind paw before inflammation and 1, 3, 5, and 7 hours after inflammation, and the swelling rate and swelling inhibition rate were calculated. Swelling rate (%), expressed as (Vt-Vn) / Vn*100, Vn and Vt represent the pa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com