Method for treating rare-earth-containing low-grade fluorite minerals
A low-grade fluorite ore technology, applied in rare earth metal compounds, chemical instruments and methods, rare earth metal sulfate, etc., can solve the problems of thorium mineral decomposition and activation, dihydrate gypsum products with excessive radioactivity, and inability to decompose fluorine-containing minerals, etc. , to ensure the effect of recycling
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
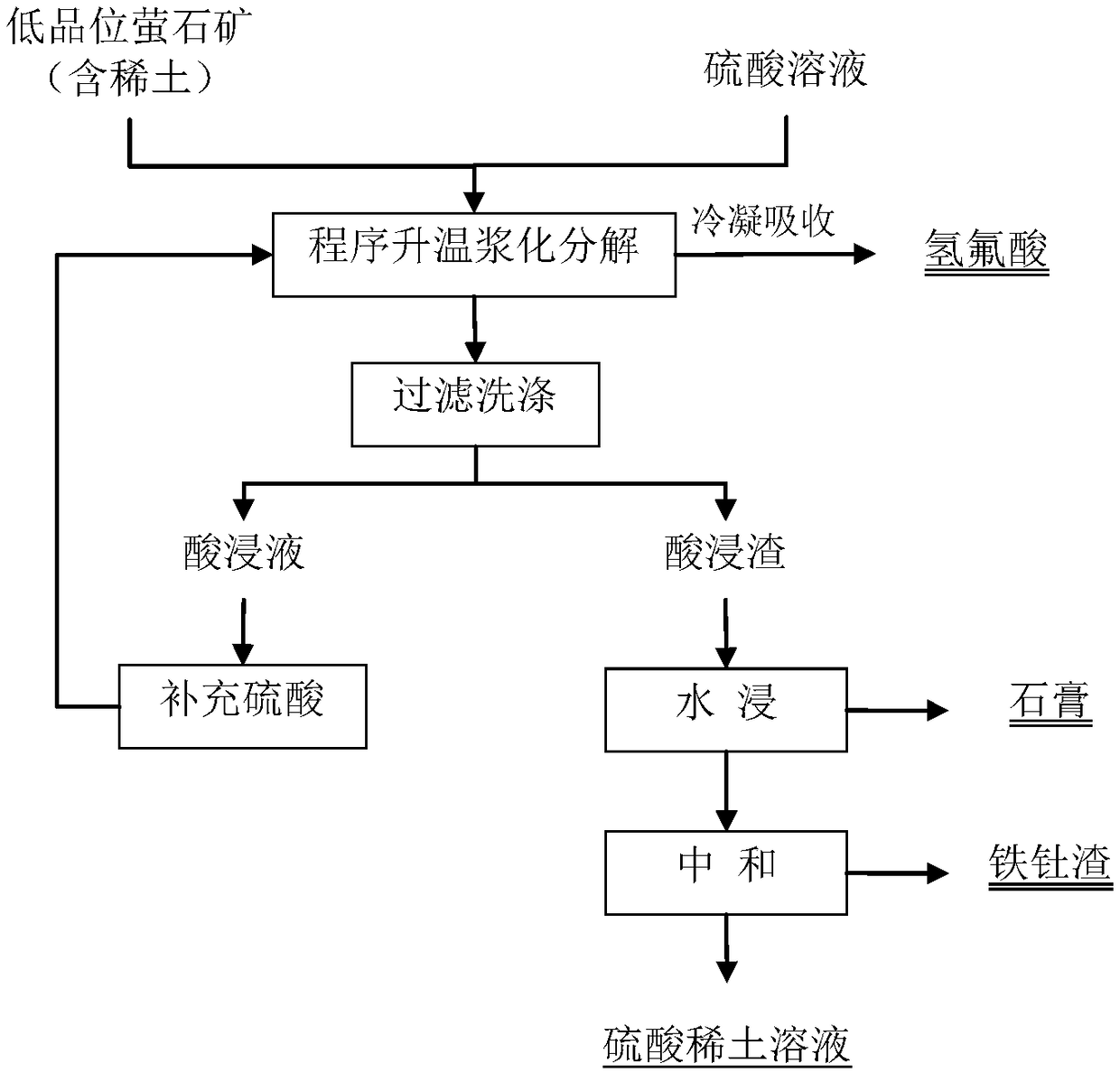
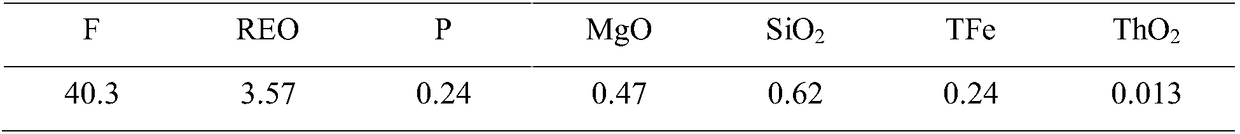
[0025] Select low-grade fluorspar ores containing rare earths (including CaF 2 80.2%, REO-containing 4.9%), get 100g of the above minerals and 714g initial mass percent concentration of 70% sulfuric acid solution mixed (sulfuric acid and mineral mass ratio is 5:1), the first step reaction temperature control 100 ~ 110°C, stirring and reacting for 90 minutes, the second step reaction temperature control 150-160°C, stirring and reacting for 30 minutes, the tail gas is condensed and absorbed by the tail gas system to obtain hydrofluoric acid. After the reaction is finished, filter and wash, filter the residue with 200ml of water for leaching, filter and wash to obtain water extract and gypsum, and neutralize and remove impurities in the water extract to obtain rare earth sulfate solution and iron thorium slag. After testing, the decomposition rate of F is 98.3%, the decomposition rate of REO is 98.6%, and the radioactivity of the obtained gypsum is 5.7×10 2 Bq / kg. The acidity o...
Embodiment 2
[0028] Select low-grade fluorspar ores containing rare earths (including CaF 2 84.7%, containing REO is 3.6%), get 100g of the above minerals and 500g initial mass percent concentration of 60% sulfuric acid solution mixed (sulfuric acid and mineral weight ratio is 3:1), the first step reaction temperature control 110 ~ 120°C, stirring and reacting for 60 minutes, the second step reaction temperature control 120-130°C, stirring and reacting for 60 minutes, the tail gas is condensed and absorbed by the tail gas system to obtain hydrofluoric acid. After the reaction is finished, filter and wash, filter the residue with 200ml of water for leaching, filter and wash to obtain water extract and gypsum, and neutralize and remove impurities in the water extract to obtain rare earth sulfate solution and iron thorium slag. After testing, the decomposition rate of F is 99.1%, the decomposition rate of REO is 88.7%, and the radioactivity of the obtained gypsum is 4.6×10 2 Bq / kg. The acid...
Embodiment 3
[0031] Select low-grade fluorspar ores containing rare earths (including CaF 2 87.9%, containing REO is 2.2%), get 100g of the above minerals and 400g initial mass percentage concentration of 50% sulfuric acid solution and mix (the weight ratio of sulfuric acid to minerals is 2:1), the first step reaction temperature control 110~ 120°C, stirring and reacting for 30 minutes, the second step reaction temperature control 140-150°C, stirring and reacting for 40 minutes, the tail gas is condensed and absorbed by the tail gas system to obtain hydrofluoric acid. After the reaction is finished, filter and wash, filter the residue with 200ml of water for leaching, filter and wash to obtain water extract and gypsum, and neutralize and remove impurities in the water extract to obtain rare earth sulfate solution and iron thorium slag. After testing, the decomposition rate of F is 98.7%, the decomposition rate of REO is 99.3%, and the radioactivity of the obtained gypsum is 3.4×10 2 Bq / kg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Radioactivity | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com