Epoxide hydrolase from rhodosporidium paludigenum and application of epoxide hydrolase
A technology of marine red yeast and epoxide, applied in the field of bioengineering, can solve the problem that the theoretical yield is only 50%, and achieve large-scale industrial production and application potential and economic value, high enantioselectivity and catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Cloning of RPEH1 mature peptide cDNA sequence
[0023] (1) A pair of specific primers were designed according to the highly homologous marine red yeast (Rhodosporidium paludigenum) CBS 6565 epoxide hydrolase gene sequence, the primer sequence is as follows:
[0024] RpEH1-F: 5'-CATATGGCTGCCCATTCCTTTACTGC-3', containing Nde I restriction site
[0025] RpEH1-R: 5'-GAATTCTCAGGCCTGGTTCGACAGCAA-3', containing EcoR I restriction site
[0026] The total RNA of R.paludigenum obtained by screening was extracted, and RT-PCR was performed according to the instructions of TaKaRa RNA PCR Kit (Ver.3.0). The first strand of cDNA was synthesized by reverse transcription using Oligo dT-Adaptor Primer as a primer. The first round of PCR amplification was performed with RpEH1-F and M13Primer M4 as primers. The PCR conditions were: 94°C for 5 min; 94°C for 30 s, 51°C for 30 s, 72°C for 2 min, 30 cycles; 72°C for 10 min. The second round of PCR amplification was carried out usi...
Embodiment 2
[0027] Example 2 Expression of RPEH1 mature peptide gene in E.coli BL21(DE3)
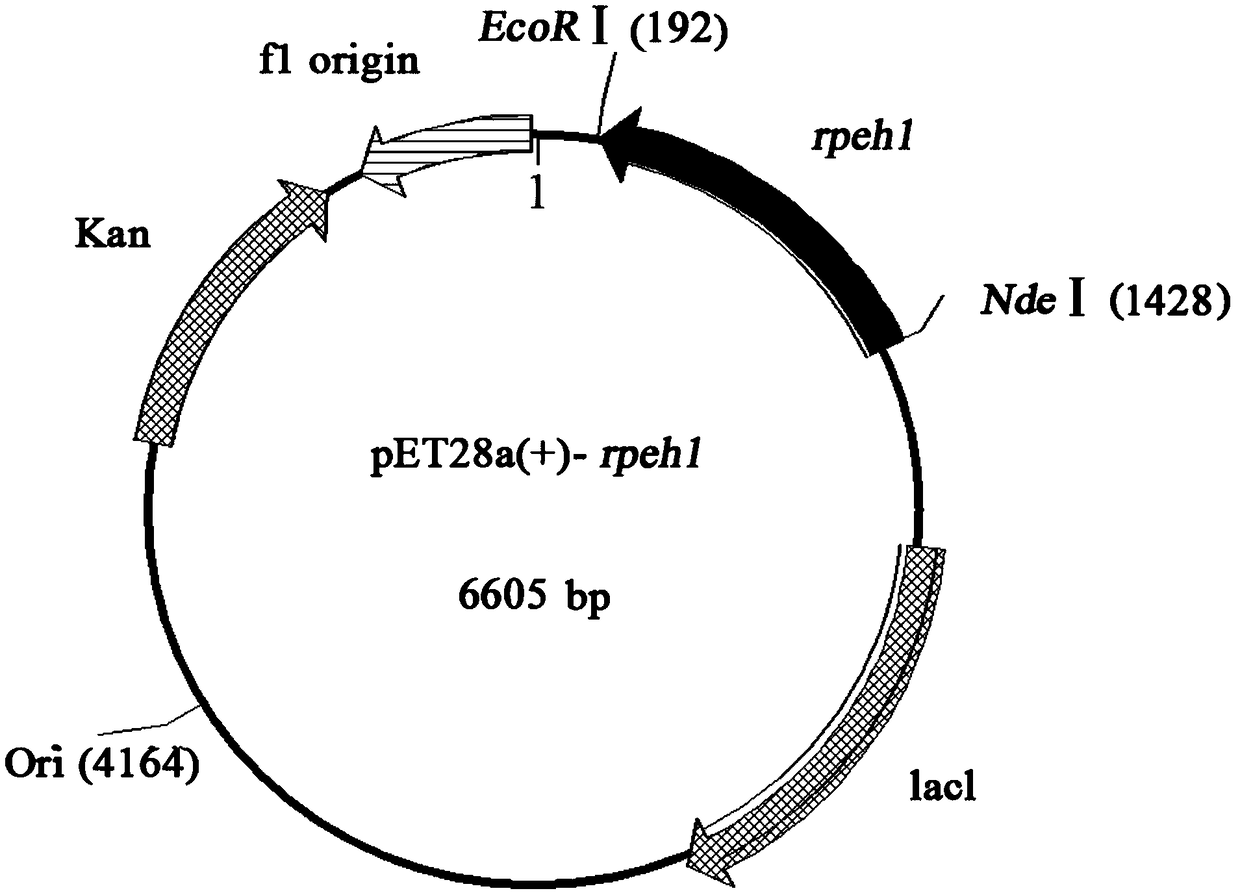
[0028] The pUCm-T-Rpeh1 and pET-28a(+) plasmids with correct sequencing results were double-digested with Nde I and Not I, and the digested products recovered from rubber tapping were ligated under the action of T4 DNA ligase to obtain the recombinant plasmid pET -28a(+)-Rpeh1, and sequenced the recombinant plasmid. Transform pET-28a(+)-Rpeh1 into E.coli BL21(DE3), and after screening on the resistance plate containing Kan, pick the positive transformants and put them in 2mL of Kan-containing resistant LB liquid medium at 37°C 215r / min shaker shaking culture 14 ~ 16h. Transplanted in 30mL of the same medium at 1% inoculum size, cultured on a shaker at 215r / min at 37°C until mid-logarithmic growth (OD 600 =0.6-0.8), adding IPTG with a final concentration of 150 μM, and inducing culture at 16° C. at 215 r / min for 10 h. The fermentation broth was centrifuged to collect the bacterial cells, washed tw...
Embodiment 3
[0029] Example 3 Application of RPEH1
[0030] The whole cell freeze-dried enzyme powder obtained by the method in Example 2 was resuspended in 50 mM pH7.2 phosphate buffer, so that the cell concentration of the resuspended bacteria was 100 mg / mL. Add 100 μL of the aforementioned resuspended bacteria solution, 45.6 μL of styrene oxide stock solution, and 854.4 μL of 50 mM phosphate buffer saline at pH 7.2 to a 2 mL EP tube. React at 25°C and 220rpm, take samples regularly for gas chromatographic analysis to calculate the conversion rate and ee p value. The result shows that under the final concentration of 10mg / mL bacterial concentration, reacted for 6 hours, the substrate styrene oxide was completely hydrolyzed, and the conversion rate reached 100%, ee p to 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com