Sphingosine kinase 1 and its fusion protein and application
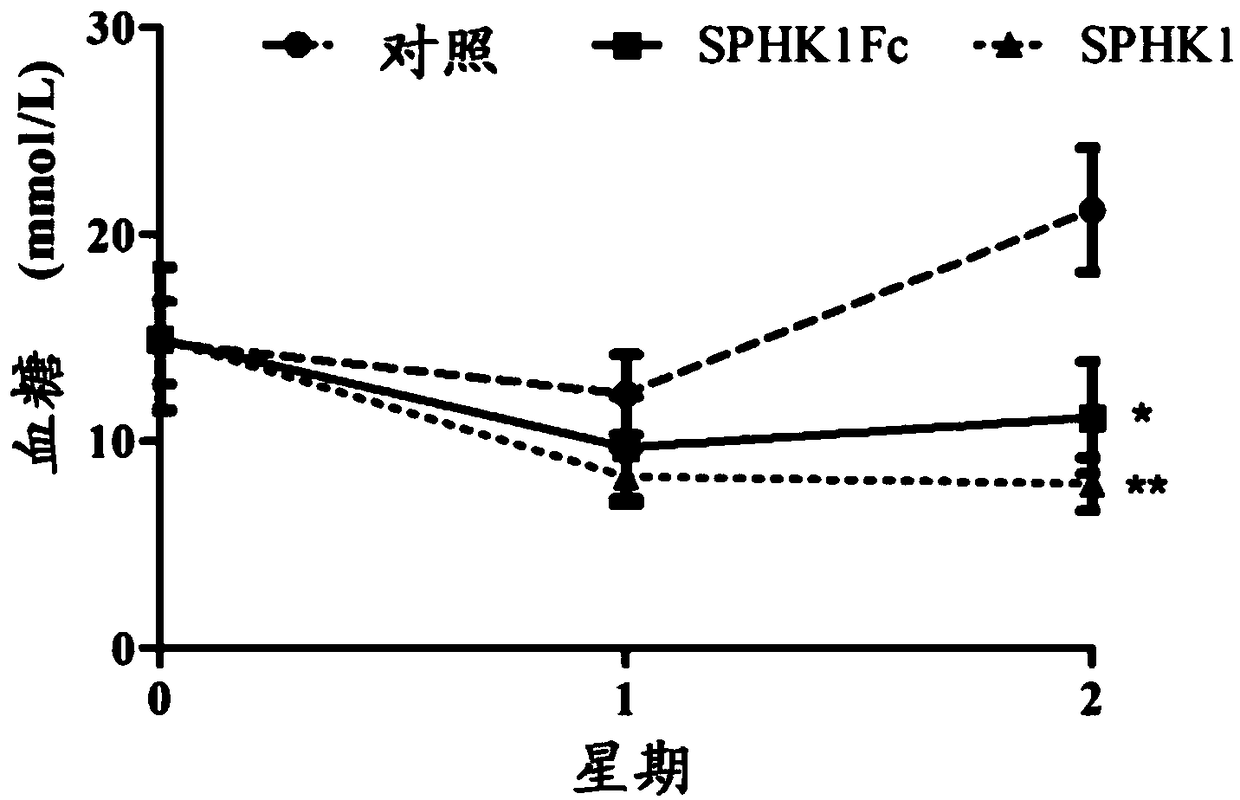
A technology of sphingosine kinase and fusion protein, which is applied in the field of biopharmaceuticals, can solve the problems of drug resistance and long-term medication, and achieve weight loss and significant hypoglycemic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
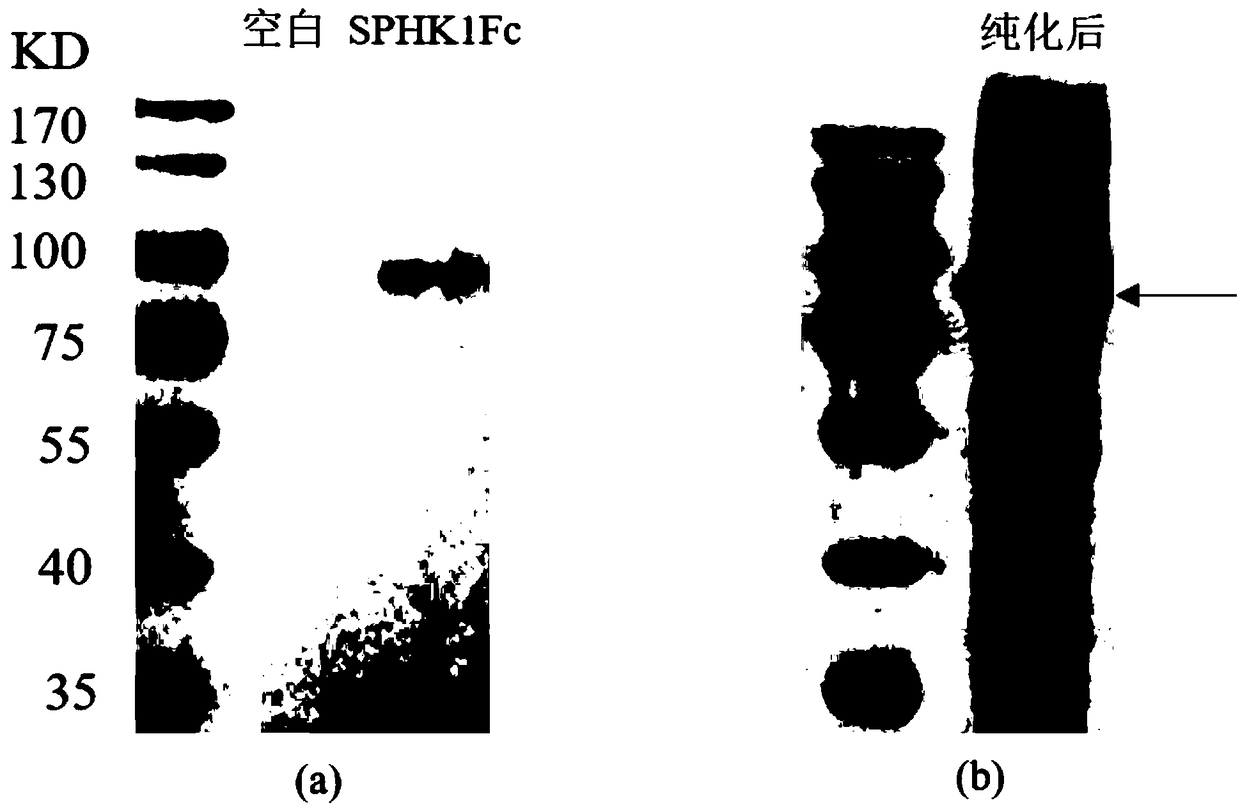
[0038] Example 1 Preparation of fusion protein SPHK1-Fc
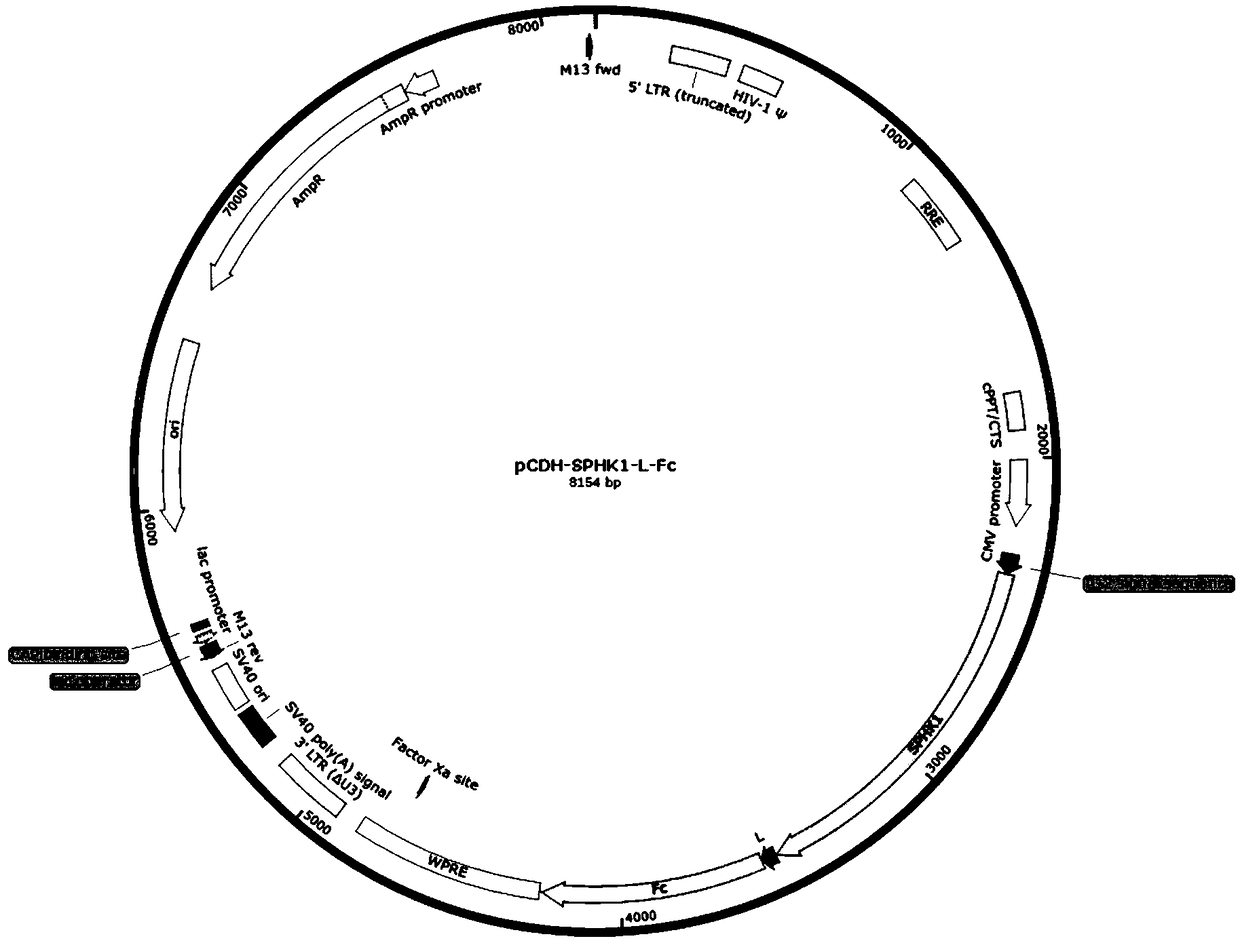
[0039]1. Construction of lentiviral expression vector pCDH-SPHK1-L-Fc containing fusion protein SPHK1-Fc
[0040] Wherein, sphingosine kinase 1 or its active amino acid sequence comprises such as SEQ ID NO: 1;
[0041] MDPAGGPRGVLPRPCRVLVLLNPRGGKGKALQLFRSHVQPLLAEAEISFTLMLTERRNHARELVRSEELGRWDALVVMSGDGLMHEVVNGLMERPDWETAIQKPLCSLPAGSGNALAASLNHYAGYEQVTNEDLLTNCTLLLCRRLLSPMNLLSLHTASGLRLFSVLSLAWGFIADVDLESEKYRRLGEMRFTLGTFLRLAALRTYRGRLAYLPVGRVGSKTPASPVVVQQGPVDAHLVPLEEPVPSHWTVVPDEDFVLVLALLHSHLGSEMFAAPMGRCAAGVMHLFYVRAGVSRAMLLRLFLAMEKGRHMEYECPYLVYVPVVAFRLEPKDGKGVFAVDGELMVSEAVQGQVHPNYFWMVSGCVEPPPSWKPQQMPPPEEPL;
[0042] Its coding nucleotide sequence is shown in SEQ ID NO:5;
[0043] ATGGACCCAGCGGGCGGCCCCCGGGGCGTGCTCCCGCGGCCCTGCCGCGTGCTGGTGCTGCTGAACCCGCGCGGCGGCAAGGGCAAGGCCTTGCAGCTCTTCCGGAGTCACGTGCAGCCCCTTTTGGCTGAGGCTGAAATCTCCTTCACGCTGATGCTCACTGAGCGGCGGAACCACGCGCGGGAGCTGGTGCGGTCGGAGGAGCTGGGCCGCTGGGACGCTCTGGTGGTCATGTCTGGAGACGGGCTGAT...
Embodiment 2
[0066] Example 2 Preparation of Lentiviral Particles Carrying Plasmid pCDH-SPHK1-L-Fc
[0067] Inoculate 293T cells (Lab217 Embryo Engineering Laboratory of Northeast Agricultural University) with a cell confluence of more than 90% into 150mm culture dishes, and inoculate 1.2×10 cells per dish. 7 1 cell, add 20ml DMEM medium containing 10% FBS, 37°C, 5% CO 2 Cultivate in saturated humidity. 2 hours before transfection, the original medium was discarded and replaced with 18ml serum-free DMEM medium. Mix the p-SPHK1-L-Fc plasmid prepared above with lentiviral-packaged helper plasmids pHelper1 and pHelper2 (Lab217 Embryo Engineering Laboratory of Northeast Agricultural University) in equal proportions, and refer to Lipofectamin 2000 transfection kit (purchased from Invitrogen) instructions to transfect 293T cells. Discard the supernatant containing the transfection mixture 6-8 hours after transfection, add 20ml of new DMEM medium containing 5% FBS to each dish, and store at ...
Embodiment 3
[0068] Example 3 Screening and verification of lentivirus infection of CHO-S cells and positive monoclonal
[0069] 3.1 Lentivirus infection of CHO-S cells
[0070] Suspended FreeStyle CHO-S cells (purchased from Thermo scientific company) were mixed with 2×10 5 Cells / mL were inoculated in a 125 mL shake flask (purchased from Corning) containing 30 mL of CD-SFM medium (CD FortiCHO medium+8mM glutamine+1×HT supplement, both purchased from Thermo scientific company), 120rpm, 8% CO2, cultured at 37°C until the logarithmic growth phase, the cells were diluted with CD-SFM medium to 4×10 4 cells / mL of cell suspension. Take 0.5 mL of cell suspension, add the above-mentioned lentiviral particles according to the multiplicity of infection (MOI) of 80, and centrifuge at 32°C and 800 g for 30 min. Discard the supernatant, re-add 0.5mL CD-SFM medium to resuspend the cells, transfer to a 24-well plate, and store at 37°C, 5% CO 2 After culturing for 48-72 hours under certain condition...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com