Blood additive
An additive, blood technology, applied in the direction of blood/immune system cells, cell culture active agents, biochemical equipment and methods, etc., can solve the problems of stimulating hemolysis, easy activation of platelets, free nucleic acid degradation, etc., to achieve strong anti-hemolysis ability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The blood additive provided by this embodiment, every 100mL comprises the following components (formulation 1):
[0050]
[0051] The present embodiment provides the following Cl-containing additives, as a control, each 100mL includes the following components:
[0052]
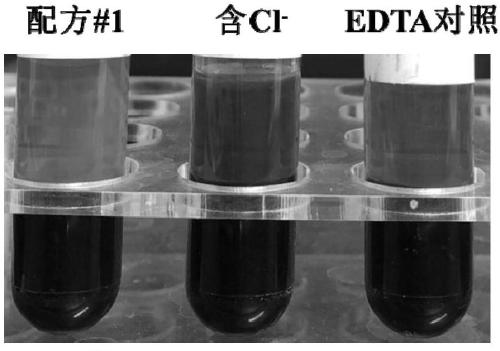
[0053] According to above-mentioned formula, weigh corresponding reagent respectively, be dissolved in 100mM phosphate buffer solution and be made into 100mL formula 1 additive of the present invention, each blood collection tube contains this additive 285.7 μ L, prepare Cl- blood collection tube with the same method, simultaneously with common EDTA blood collection tube for comparative test. Specific steps are as follows:
[0054] 1. Use EDTA blood collection tubes, blood collection tubes containing formula 1 additives and Cl- additives to collect blood respectively. The blood collection volume of each tube is 10mL. mix;
[0055] 2. Store at room temperature, observe the hemolysis of the blood...
Embodiment 2
[0058] The blood supplement provided by this embodiment, every 100mL comprises the following components (formulation 2):
[0059]
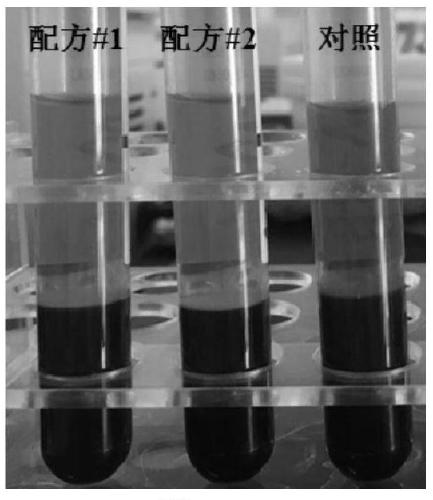
[0060] Additives were prepared according to the above formula, and a comparative test was carried out with common EDTA blood collection tubes. Specific steps are as follows:
[0061] 1. Use EDTA blood collection tubes, blood collection tubes containing the additive of formula 1 in Example 1, and blood collection tubes containing the additive of formula 2 in Example 2 to collect blood respectively. The blood collection volume of each tube is 10 mL, and immediately after blood collection, slowly turn the blood collection tube upside down 10 times , so that the blood and additives are fully mixed;
[0062] 2. After being transported at room temperature for 4 days, the blood collection tubes were centrifuged at 2000g for 10 minutes to separate the plasma, and the hemolysis of each blood collection tube was observed and compared.
[0063] experime...
Embodiment 3
[0065] Using the blood additive (formula 1) of Example 1, with the common EDTA blood collection tube as a control, carry out the normal temperature transportation experiment, the specific steps are as follows:
[0066] 1. The tumor cell line H1975 (EGFR T790M mutation-positive cell line) was cultured in RMPI 1640 medium (containing 10% fetal bovine serum) at 37°C and 5% CO2 for 3 days, and then transferred to a clean centrifuge tube;
[0067] 2. Centrifuge at 2000g for 10min, transfer the culture supernatant to a new clean centrifuge tube;
[0068] 3. Use the blood collection tube containing the additive of the present invention and the common EDTA blood collection tube to collect the peripheral blood of 3 volunteers respectively, each tube collects 10 mL, and immediately turn the blood collection tube upside down slowly for 10 times;
[0069] 4. Take the supernatant of the culture medium in step 2, vortex to mix, add 50 μL to each tube of 10 mL of blood in step 3, and slowly ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| alcoholysis degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com