Construction of stable cell line carrying orthogonal tRNA/aminoacyl tRNA synthetase
A cell line and synthetase technology, applied in genetically modified cells, retroRNA viruses, cells modified by introducing foreign genetic material, etc., can solve problems such as the bottleneck of industrial application of gene codon expansion technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
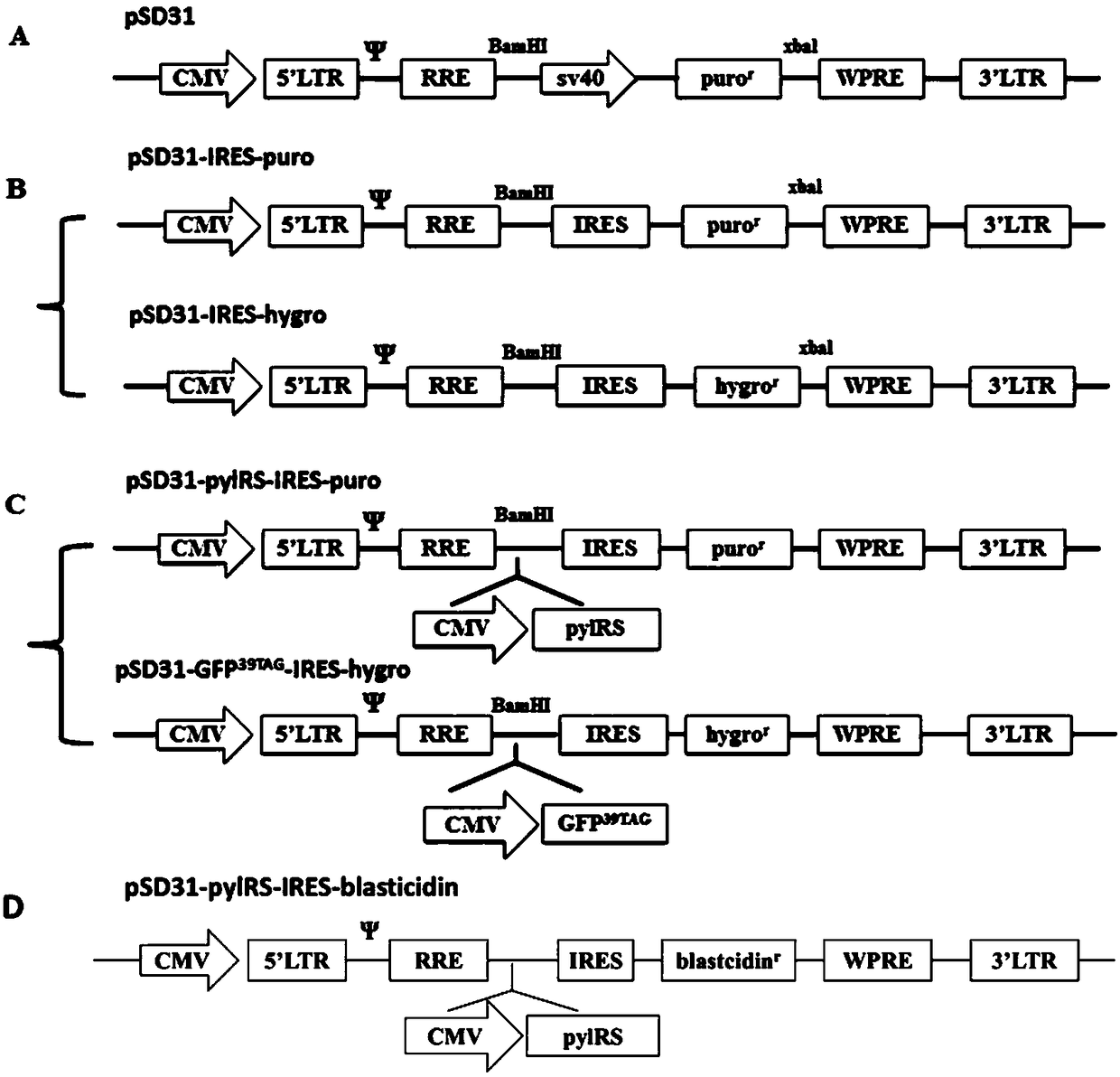
[0057] Example 1 Construction and acquisition of double lentiviral vectors
[0058] 1. Obtaining the Vector Skeleton
[0059] The backbone of the double lentiviral vector is the lentiviral vector pSD31 (Zhang Jing. et al. RNA, 2007, 13: 1375-1383.), wherein the sv40 promoter promotes the puromycin resistance gene protein puro Rexpression.
[0060] 2. Primer design for SOE PCR
[0061] The inventors used SOE PCR to splicing the DNA fragments of the internal ribosome entry sequence (IRES) and the puromycin resistance gene / hygromycin B resistance gene to obtain IRES-puro and IRES- hygro fragment, the specific primers are shown in the table below.
[0062] Table 1: List of SOE PCR primers
[0063]
[0064] 3. Transformation of Lentiviral Vectors
[0065] On the basis of pSD31, the sv40 promoter and puromycin resistance gene fragments were replaced by IRES-puro and IRES-hygro fragments by BamHI and xbal double digestion, respectively, so as to obtain puromycin resistance an...
Embodiment 2
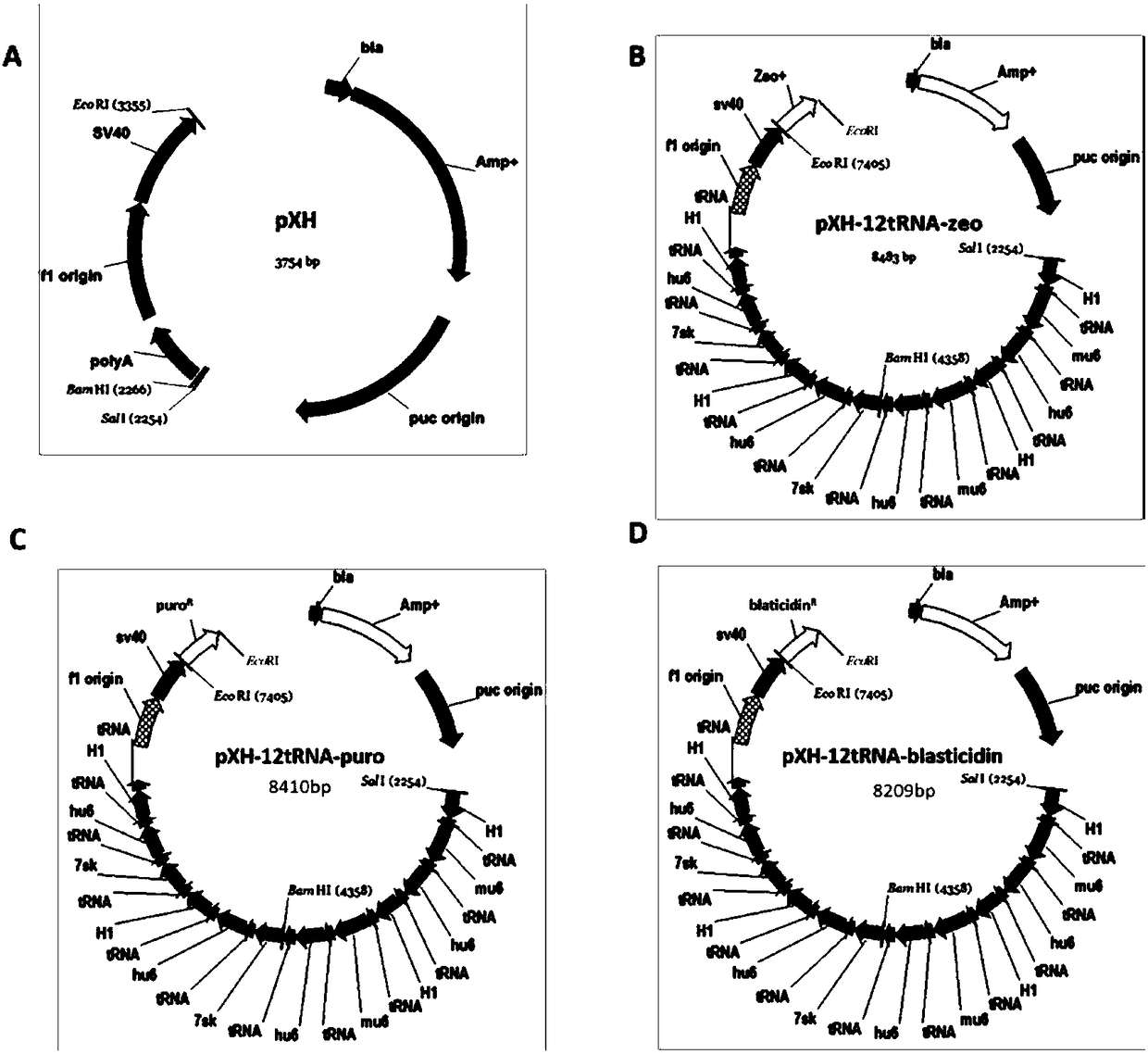
[0068] Example 2 Construction and acquisition of pXH-12tRNA-blasticidn vector
[0069] In order to ensure the expression level of tRNA, it is necessary to clone multiple copies of the tandemly expressed promoter-tRNA into a suitable vector. In the present invention, the pXH blank vector is used as the backbone, and the zeomycin-polyA sequence is introduced into the back of the SV40 promoter through the EcoRI restriction site, so that it has bleomycin resistance. After that, 12 copies of the promoter-tRNA sequence were cloned into the pXH-zeo vector using the SalI restriction site. In order to avoid the probability of recombination of the repeated sequences, 4 different tRNA promoters were used: 7sk / hu6 / H1 / mu6. Finally, the vector bjmu-12t-zeo for screening tRNA was obtained.
[0070] 1. Obtaining the Vector Skeleton
[0071] The backbone of the pXH-12tRNA-zeo vector is the vector pXH, which is a shuttle vector obtained by transforming the PUC19 vector. The pXH sequence is...
Embodiment 3
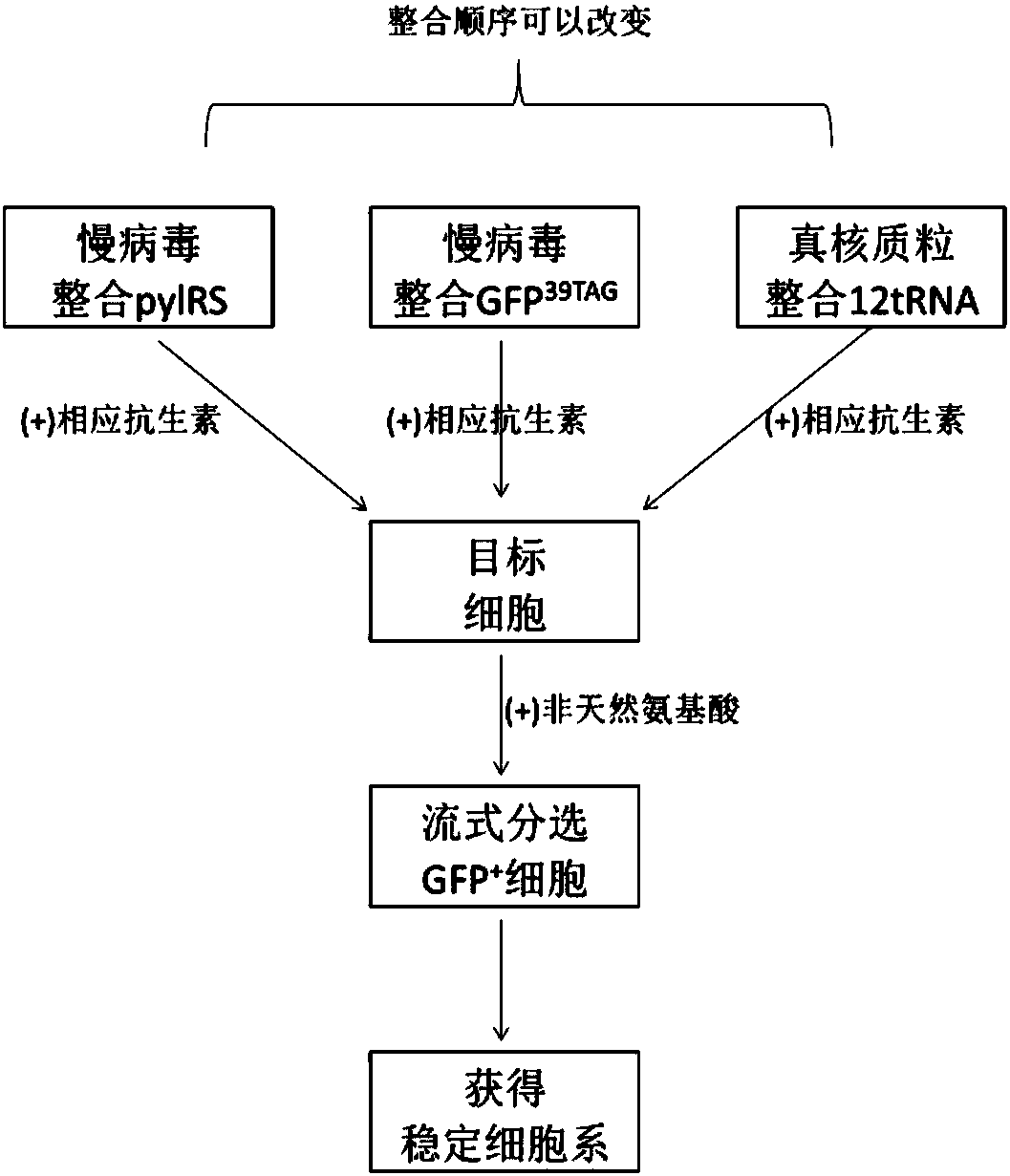
[0083] Example 3 Screening of stable cell lines
[0084] (1) Packaging and transduction of lentivirus, including the following steps:
[0085] a. HEK 293T cell plating: use medium A, components (DMEM+10% FBS, 1×NEAA, without sodium pyruvate), cell digestion count, and the number of cells seeded in each well of a six-well plate is 4×10 5 cells / well.
[0086] b. Lentiviral packaging: transfection is carried out at a cell density of 70% to 80%. The formulations of plasmids and transfection reagents are shown in Table 3-1. 6 hours after transfection, medium B (DMEM + 3% FBS, 1 x NEAA, With Sodium Pyruvate) was changed. Continue to cultivate. The virus solution was harvested at 48 hours and 72 hours after transfection, and filtered through a PVDF membrane syringe filter with a pore size of 0.45 μm.
[0087] Table 3-1. Plasmid ratio for lentiviral packaging
[0088] Plasmids / Transfection Reagents
Dosage per well
Opti-MEM
200μl
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com