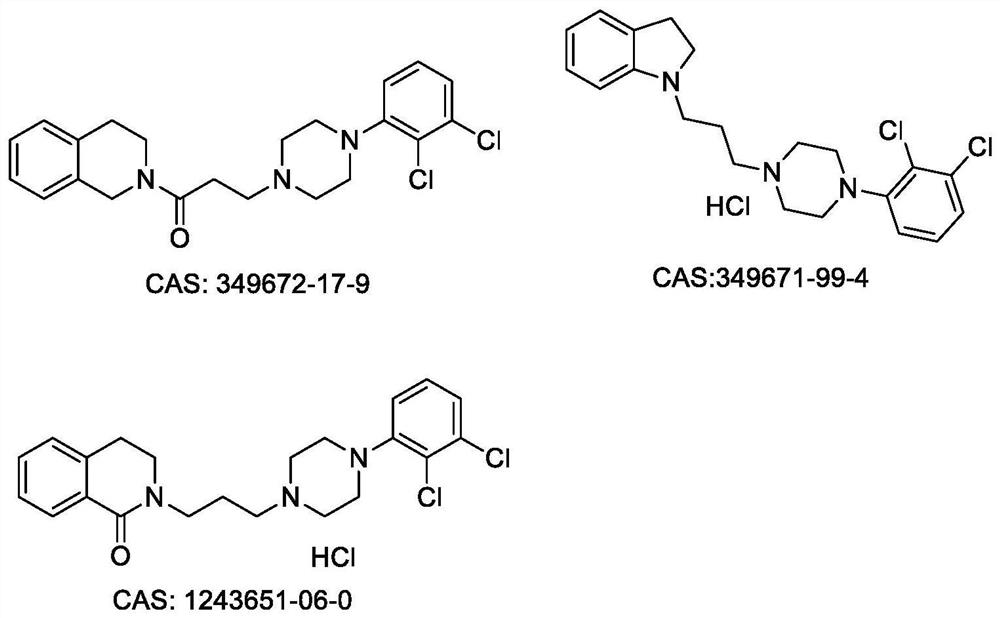
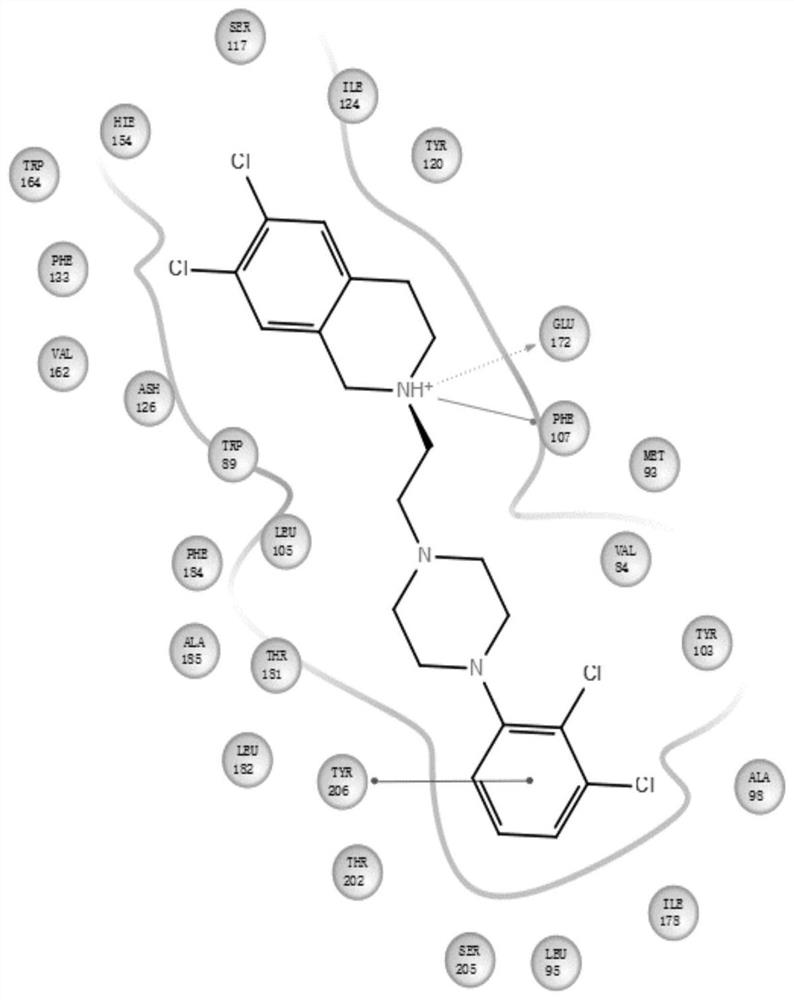
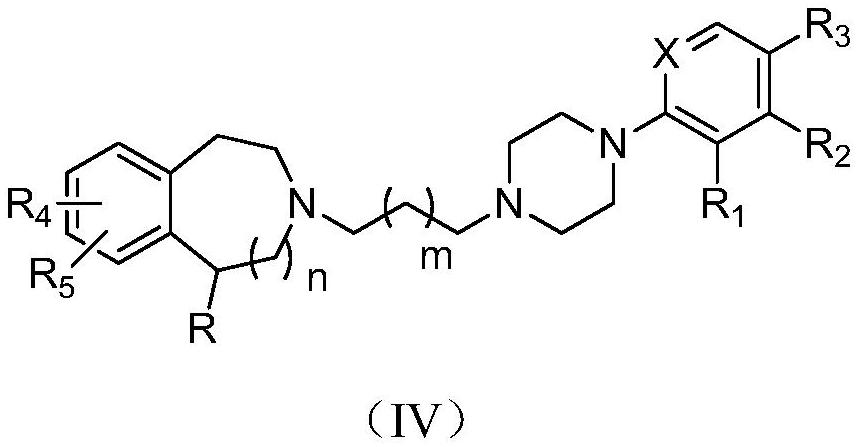
Benzozaalkylarylpiperazine derivatives and their application in the preparation of medicines
A technology for benzazepines and derivatives, which is applied to benzazepine alkylarylpiperazine derivatives and the application field in the preparation of medicines, can solve different structural characteristics, reduce adverse reactions, pharmacological action mechanisms and To treat problems such as different indications, to achieve the effect of high safety index, small toxic and side effects, and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] 1. Preparation of intermediate compound III-1
[0092]
[0093] Intermediate III-1-4: Preparation of methyl 2-bromo-3-(3,4-dichlorophenyl)propionate
[0094] Add 16ml of 48% hydrogen bromide to 100ml of acetone solution, slowly add 3,4-dichloroaniline (8.1g, 50mmol), keep the internal temperature at -5°C and slowly add 100ml of an aqueous solution of 4.2g of sodium nitrite for 30min The inner drop is complete. Then add 43g of methyl acrylate and 7.5mg of CuBr powder to it, remove the ice bath, stir for 30min after the temperature rises slowly to 25°C, evaporate the solvent, distribute with 50ml of water and dichloromethane each, and invert the dichloromethane layer with water washed, dried over anhydrous magnesium sulfate, filtered, and the filtrate was evaporated to dryness to obtain 14.2 g of the product.
[0095] Intermediate III-1-3: Preparation of 3,4-dichlorophenylpropionic acid
[0096] Dissolve 13.1g of methyl 2-bromo-3-(3,4-dichlorophenyl)propionate in 80...
Embodiment 1
[0123] 2-(3-(4-(2,3-dimethylphenyl)piperazin-1-yl)propyl)-1,2,3,4-tetrahydroisoquinoline (IV-1) disalt Preparation of acid salt and 2-(3-(4-(2,3-dimethylphenyl)piperazin-1-yl)propyl)-1,2,3,4-tetrahydroisoquinoline (IV -1) Preparation of hydrobromide
[0124] Take the compound 1-(2,3-dimethylphenyl)piperazine hydrochloride (0.1mol, 1.0eq.) and dissolve it in 150ml of dichloromethane, and add NaOH aqueous solution (5.2g, 50ml) dropwise to the above solution , stirred at room temperature for 0.5h, stood to separate layers, extracted the aqueous phase with 30ml of dichloromethane, combined the organic phases, concentrated to obtain an oil, dissolved in 30ml of acetone, added 1-bromo-3-chloropropane (0.11mol, 1.1equiv. ), add 18ml of 25% NaOH aqueous solution, stir at room temperature and react for 12h, stop stirring, let the layers stand, extract the water layer with dichloromethane, combine the organic phases, wash with saturated brine, separate the liquids, and dry the organic ...
Embodiment 2
[0133] 2-(3-(4-(2,3-dichlorophenyl)piperazin-1-yl)propyl)-1,2,3,4-tetrahydroisoquinoline (IV-2) dihydrochloride salt preparation
[0134] Take compound 1-(2,3-dichlorophenyl)piperazine hydrochloride (0.1mol, 1.0eq.), add 150ml of dichloromethane to dissolve, and add NaOH aqueous solution (5.2g, 50ml) dropwise to the above solution , stirred at room temperature for 0.5h, stood to separate layers, extracted the aqueous phase with 30ml of dichloromethane, combined the organic phases, concentrated to obtain an oil, dissolved in 30ml of acetone, added 1-bromo-3-chloropropane (0.11mol, 1.1equiv. ), add 18ml of 25% NaOH aqueous solution, stir at room temperature and react for 12h, stop stirring, let the layers stand, extract the water layer with dichloromethane, combine the organic phases, wash with saturated brine, separate the liquids, and dry the organic phases through anhydrous sodium sulfate. Filtration, concentration, purification by silica gel column chromatography (eluent: d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com