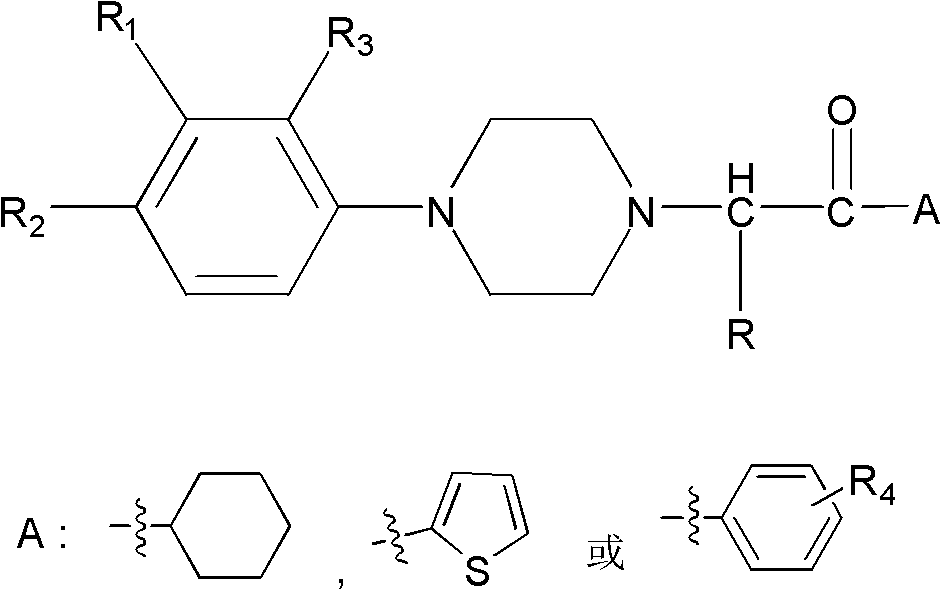
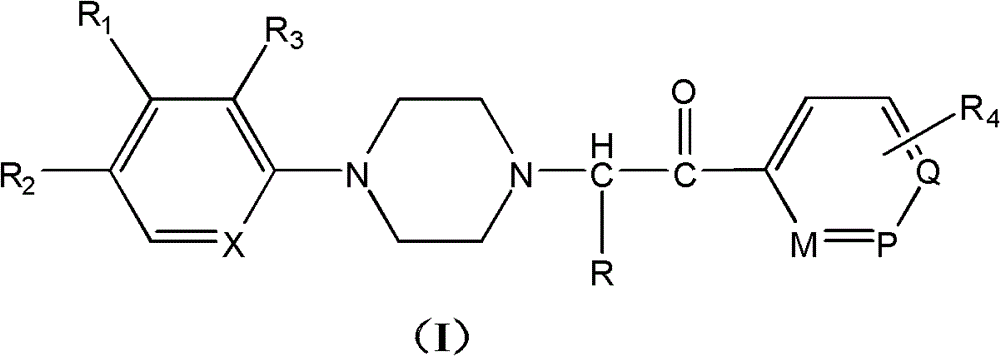
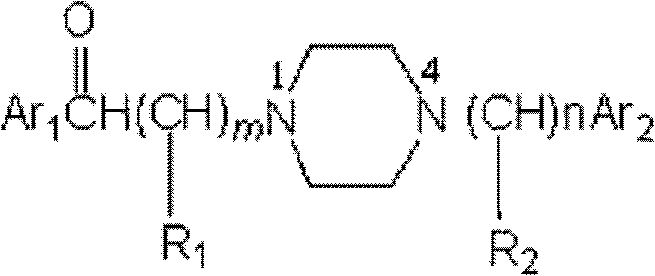Substituted arylpiperazine aryl alkanone derivatives and their application in the preparation of analgesic drugs
A technology of arylpiperazine and aralkylone, which is applied in the field of arylpiperazine aralkylone compounds, can solve problems such as reduction of gastric peristalsis, and achieve the effect of strong analgesic effect and strong anti-pain writhing reaction effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] 1-(pyridin-2-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone (I-1) hydrochloride, hydrobromide and Sulfate preparation
[0082] 1) Intermediate: Synthesis of 2-bromo-1-(pyridin-2-yl)ethanone hydrobromide
[0083] 1-(pyridin-2-yl)ethanone (6.00g, 0.05mol) was added in 55ml of glacial acetic acid, then bromine (8.80g, 0.055mol) and 40% hydrobromic acid (10.20g, 0.05mol), heated up to 75°C for 3.0h, stopped heating, cooled in an ice-water bath, filtered, and recrystallized with a mixed solvent of ethyl acetate and methanol (weight ratio 5:1) to obtain white solid 2-bromo-1-( Pyridin-2-yl)ethanone hydrobromide 12.4g, yield 88.2%.
[0084] ESI-MS[M+H]+: m / z199.96
[0085] 2) Synthesis of 1-(pyridin-2-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone (I-1) hydrochloride
[0086] m-Trifluoromethylphenylpiperazine hydrochloride (2.67g, 0.01mol), 2-bromo-1-(pyridin-2-yl)ethanone hydrobromide (2.81g, 0.011mol) and anhydrous Potassium carbonate (5.52g, 0...
Embodiment 2
[0098] Preparation of 1-(pyridin-3-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone (I-2) hydrochloride 1) intermediate : Synthesis of 2-bromo-1-(pyridin-3-yl)ethanone hydrobromide
[0099] 1-(pyridin-3-yl)ethanone (6.00g, 0.05mol) was added in 55ml of glacial acetic acid, then bromine (8.80g, 0.055mol) and 40% hydrobromic acid (10.20g, 0.05mol), heated to 75°C for 3.0h, stopped heating, cooled in an ice-water bath, filtered, and recrystallized with a mixed solvent of ethyl acetate and methanol (5:1 by weight) to obtain off-white solid 2-bromo-1- (Pyridin-3-yl)ethanone hydrobromide 13.1 g, yield 93.2%.
[0100] ESI-MS[M+H]+: m / z199.96
[0101] 2) Synthesis of 1-(pyridin-3-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone (I-2) hydrochloride
[0102] m-Trifluoromethylphenylpiperazine hydrochloride (2.67g, 0.01mol), 2-bromo-1-(pyridin-3-yl)ethanone hydrobromide (2.81g, 0.011mol) and anhydrous Potassium carbonate (5.52g, 0.04mol) was added into 30ml of DM...
Embodiment 3
[0107] Preparation of 1-(pyridin-4-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone (I-3)hydrochloride
[0108] 1) Intermediate: Synthesis of 2-bromo-1-(pyridin-4-yl)ethanone hydrobromide
[0109] 1-(pyridin-3-yl)ethanone (2.42g, 0.02mol) was added in 25ml of glacial acetic acid, then bromine (3.52g, 0.022mol) and 40% hydrobromic acid (4.08g, 0.02mol), heated to 75 ° C for 3.0 h, stopped heating, cooled in an ice-water bath, filtered, and recrystallized with a mixed solvent of ethyl acetate and methanol (5:1 by weight) to obtain off-white solid 2-bromo-1- (Pyridin-4-yl)ethanone hydrobromide 4.04g, yield 71.9%.
[0110] ESI-MS[M+H] + : m / z199.98
[0111] 2) Synthesis of 1-(pyridin-4-yl)-2-(4-(3-(trifluoromethyl)phenyl)piperazin-1-yl)ethanone (I-3) hydrochloride
[0112] m-Trifluoromethylphenylpiperazine hydrochloride (2.67g, 0.01mol), 2-bromo-1-(pyridin-4-yl)ethanone hydrobromide (2.81g, 0.011mol) and anhydrous Potassium carbonate (5.52g, 0.04mol) was added into...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com