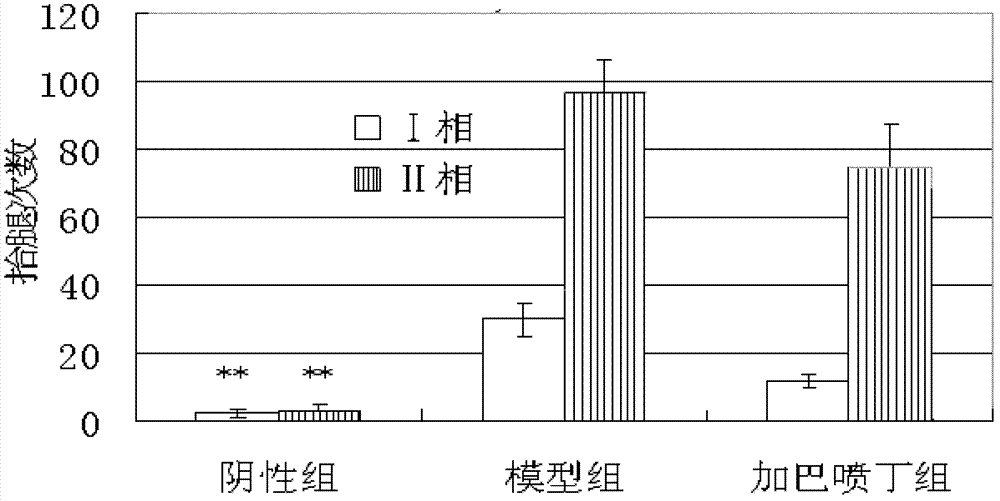
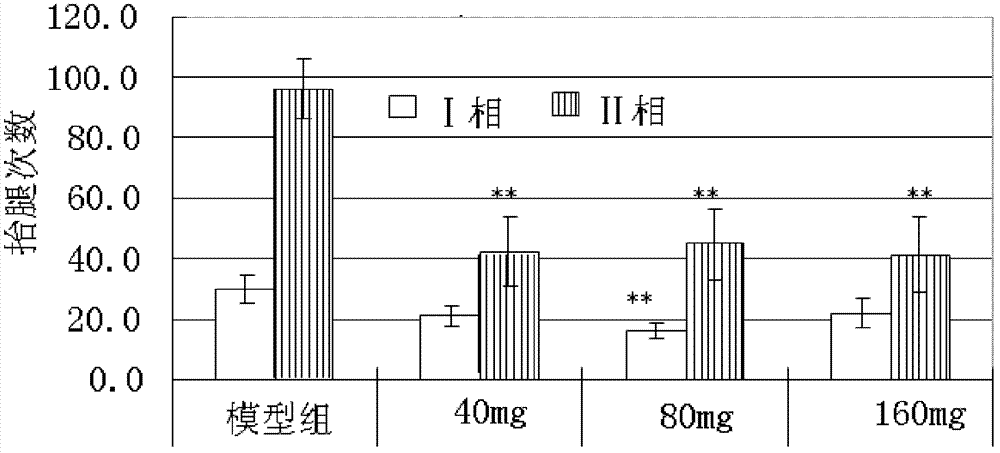
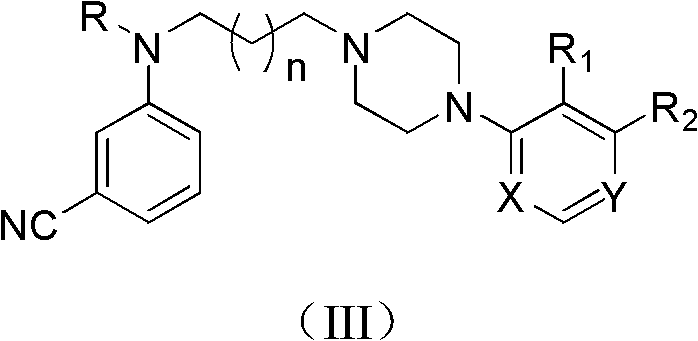
3-Cyanoaniline alkyl aryl piperazine derivative and application in preparing medicaments
A technology of cyanoanilinidine and its derivatives, which is applied in the field of 3-cyanoanilinoalkylarylpiperazine derivatives, which can solve the problems of large toxic and side effects, decreased addictive gastric motility, and slow onset of action, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0081] Preparation of intermediate compounds
[0082] 1. Preparation of 4-toluenesulfonic acid (2-((3-cyanophenyl)methylamino)ethyl) ester
[0083] 1) Preparation of 3-methylaminobenzonitrile
[0084] Add 4.05g (75mmol, 0.5eq) CH to 45ml methanol 3 ONa, dissolve 17.70 g (150 mmol, 1.0 eq) of 3-aminobenzonitrile in 60 ml of methanol and add dropwise to the methanol solution of sodium methoxide. After stirring for 0.5 h at room temperature, the above reaction solution was poured into a 90 ml methanol solution of 6.30 g (210 mmol, 1.4 eq) of paraformaldehyde. After stirring the reaction at room temperature for 5.0h, add 6.00g (150mmol, 1.0eq) NaBH in batches 4 (Content 96%). The reaction was stirred at room temperature for 10 min, and the temperature was raised to reflux for 10 min. Cool the reaction solution in an ice-water bath, add 90ml 10% NaOH(aq) dropwise, stir for 5min, evaporate the methanol under reduced pressure, extract with ethyl acetate (150ml*2), combine the organic pha...
Embodiment 1
[0117] 3-((2-(4-(2-Methoxyphenyl)piperazin-1-yl)ethyl)methylamino)benzonitrile (III-1) hydrochloride, hydrobromide and sulfate Preparation
[0118] 2.90g (8.8mmol, 11eq) 4-toluenesulfonic acid (2-((3-cyanophenyl)methylamino)ethyl) ester and 1.54g (8.0mmol, 1.0eq) 2-methoxyphenyl Piperazine, 1.33g (8.0mmol, 1.0eq) potassium iodide and 4.10g
[0119] (32mmol, 4.0eq) diisopropylethylamine was added to 50ml of acetonitrile, heated and refluxed for 12h, the reaction of the raw materials was complete. The solvent was evaporated under reduced pressure, 35ml of water was added to the residue, extracted with ethyl acetate (35ml*2), separated, the organic phase was washed once with saturated brine, anhydrous Na 2 SO 4 Dry and concentrate to obtain 3.06 g of crude product as brown oil. Purified by neutral alumina column chromatography, firstly with petroleum ether: ethyl acetate=3:1 (volume ratio), and then eluted with dichloromethane as the mobile phase to obtain compound (III-1), powdery ...
Embodiment 2
[0131] Preparation of 3-(methyl(2-(4-(3-trifluoromethylphenyl)piperazin-1-yl)ethyl)amino)benzonitrile (III-2) hydrochloride
[0132] 2.79g (8.5mmol, 11eq) 4-toluenesulfonic acid (2-((3-cyanophenyl)methylamino)ethyl) ester and 1.77g (7.6mmol, 1.0eq) 3-trifluoromethylbenzene Piperazine, 1.26g (7.6mmol, 1.0eq) potassium iodide and 3.90g
[0133] (30.4mmol, 4.0eq) diisopropylethylamine was added to 46ml of acetonitrile, heated to reflux for 12h, and the reaction of the raw materials was complete. The solvent was evaporated under reduced pressure, 35ml of water was added to the residue, extracted with ethyl acetate (30ml*2), separated, the organic phase was washed once with saturated brine, anhydrous Na 2 SO 4 After drying and concentrating, 3.6 g of crude brown oily product was obtained. Purified by neutral alumina column chromatography, firstly with petroleum ether: ethyl acetate=3:1 (volume ratio), and then eluted with dichloromethane as the mobile phase to obtain compound (III-2) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com