Anti-rat CD3 recombinant immunotoxin as well as preparation method and application thereof
A technology of immunotoxin and toxin, which is applied in the biological field and can solve problems such as different half-lives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
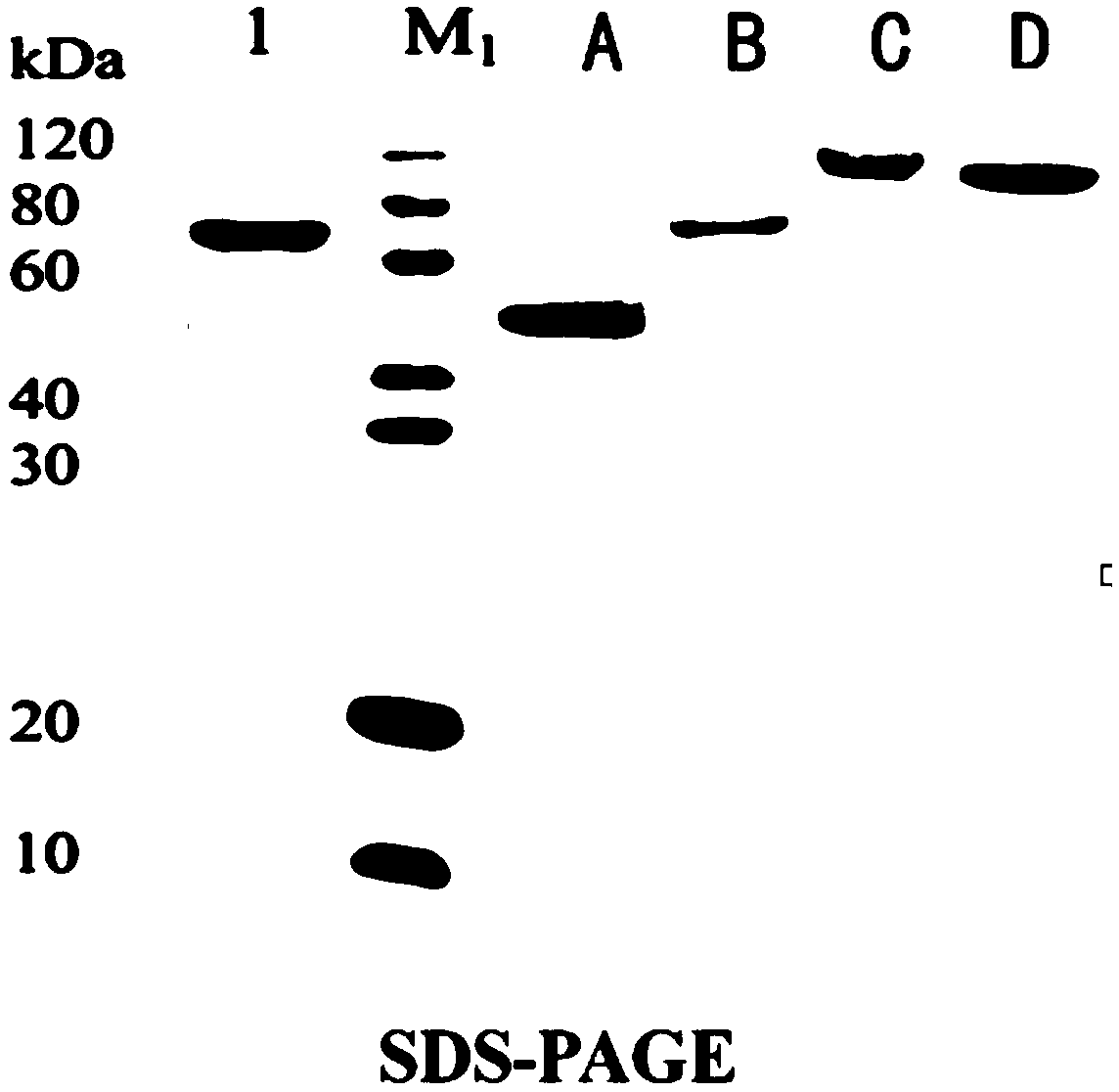
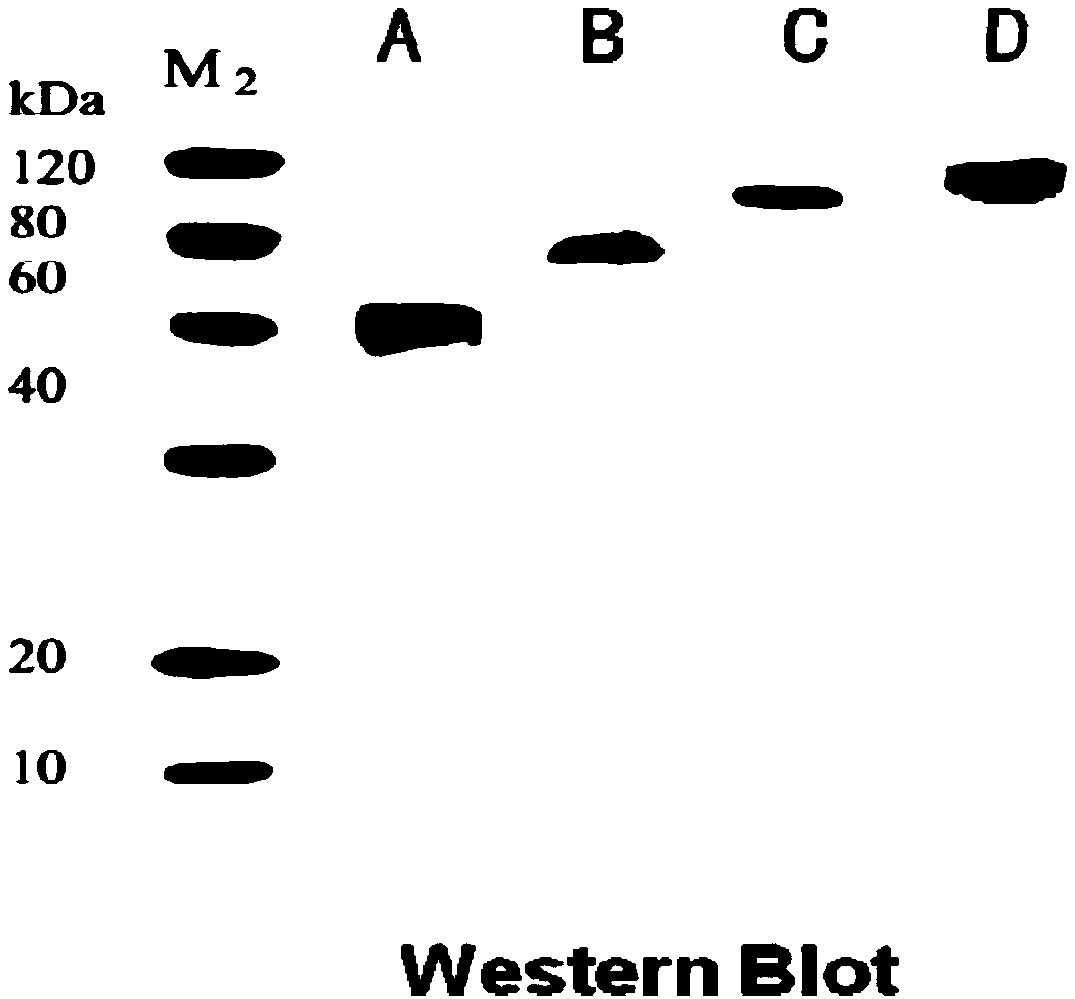
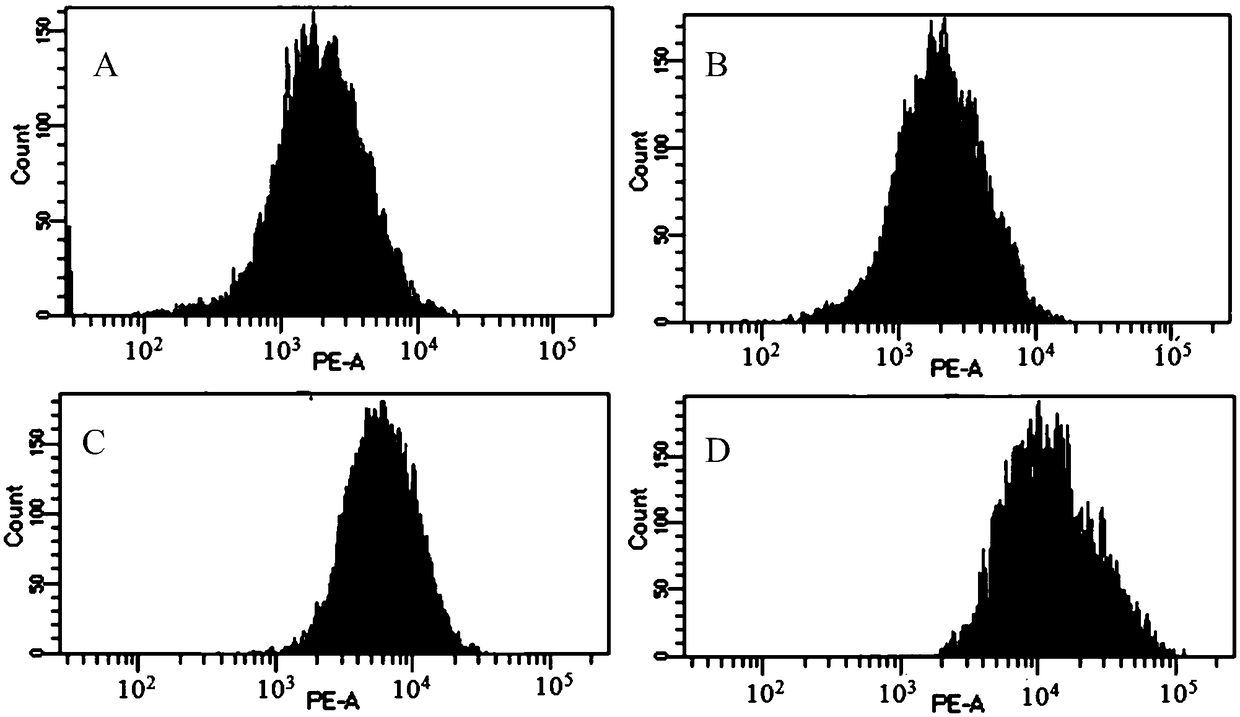
Image
Examples
Embodiment 1
[0047] (1) Expression vector construction of mouse anti-CD3 recombinant immunotoxin:
[0048] Recombinant immunotoxins: ScFv-CD3-DT390, Bi-CD3-DT390, Foldback-CD3-DT390, construct their prokaryotic expression system.
[0049] Design and optimize the codon nucleotide sequence of mouse anti-CD3 recombinant immunotoxin, see SEQ ID NO: 8, SEQ ID NO: 9, SEQ ID NO: 10; introduce KpnI restriction site and EK recognition at the 5' end respectively Site-(Asp) 4 Lys coding sequence and design start codon ATG, wherein EK recognition site-(Asp) 4 Lys coding sequence in order to excise the His-Tag of the final expressed fusion protein; design 6 histidine codons CGTCGTCGTCGTCGTCGT, stop codon TAATGA and introduce XhoI restriction site at the 3′ end, so that each construct is easy to protein Purification and detection, while removing the stop codon at the 3' end of the coding sequence.
[0050] The nucleotide sequence was synthesized by the company, then amplified by PCR technology, and m...
Embodiment 2
[0059] In vitro cell model analysis of biological characteristics of mouse anti-CD3 recombinant immunotoxin and its effect on mouse CD3 phenotype knockout
[0060] 1. In vitro cell model analysis of mouse anti-CD3 recombinant immunotoxin binding to mouse CD3 and phenotype knockout
[0061] Analysis of murine anti-CD3 recombinant immunotoxin binding and killing of murine peripheral blood mononuclear cells in vitro using healthy mouse peripheral blood cells.
[0062] (1) Prepare enough mouse peripheral blood lymphocytes and dilute them with cell culture medium to 2.0×10 6 cells / mL, 500 μL per well was added to a 24-well plate.
[0063] (2) Dilute the purified mouse anti-CD3 recombinant immunotoxin with lymphocyte culture medium to 1.0×10 -6 M, 1.0×10 -7 M, 1.0×10 -8 M and 1.0×10 -9 M.
[0064] (3) Add 50 μL of diluted mouse anti-CD3 recombinant immunotoxin or negative control into corresponding wells, and incubate in a 37° C. incubator for 48 hours.
[0065] (4) Collect c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com