Anti-EGFRvIII safe chimeric antigen receptor and preparation method thereof, NK cell modified by anti-EGFRvIII safe chimeric antigen receptor and application of anti-EGFRvIII safe chimeric antigen receptor
A chimeric antigen receptor, NK cell technology, applied in the field of genes to reduce toxic side effects and improve clinical safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
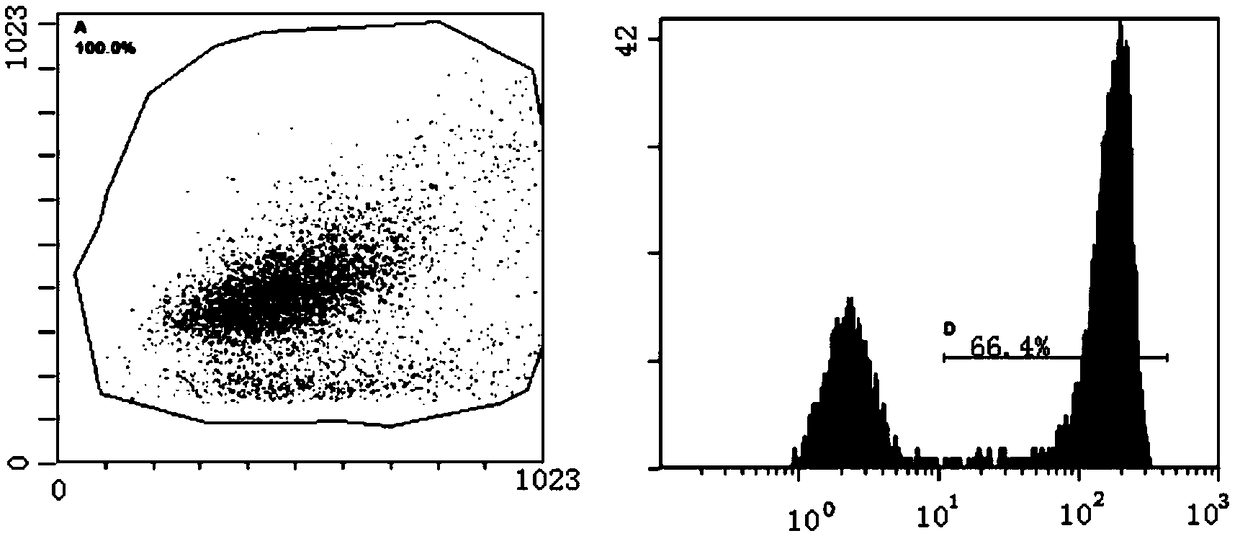
[0042] Anti-EGFRvIII safe chimeric antigen receptor, such as figure 1As shown, its nucleic acid sequence must contain a molecular switch RQR8 nucleic acid sequence, and the RQR8 nucleic acid sequence has a nucleic acid sequence as described in SEQ ID NO.9. RQR8 is composed of signal peptide nucleic acid sequence (SEQ ID NO.10), CD20 mimic epitope nucleic acid sequence (SEQ ID NO.11), CD34 mimic epitope nucleic acid sequence (SEQ ID NO.12), CD20 mimic epitope The nucleic acid sequence (SEQ ID NO.11) and the CD8 hinge transmembrane region nucleic acid sequence (SEQ ID NO.13) are sequentially concatenated, and the complete nucleic acid sequence is shown in SEQ ID NO.9. Sangon Bioengineering (Shanghai) Co., Ltd. was commissioned to synthesize the nucleotide sequence.
[0043] In this embodiment, the anti-EGFRvIII safe chimeric antigen receptor comprises the CD8leader nucleic acid artificial sequence as described in SEQ ID NO.2; the EGFRvIII scFv nucleic acid artificial sequence a...
Embodiment 2
[0046] A method for preparing an anti-EGFRvⅢ safe chimeric antigen receptor, comprising the steps of:
[0047] (1) Synthesize the entire expression cassette according to the CD8leader nucleic acid sequence, EGFRvIII scFv nucleic acid sequence, CD8α nucleic acid sequence, NKG2D nucleic acid sequence, 2B4 nucleic acid sequence, CD3ζ nucleic acid sequence, self-cleaving polypeptide T2A nucleic acid sequence and RQR8 nucleic acid sequence and insert it into the standard vector pUC Above, get pUC-Anti-EGFRvIII CAR;
[0048] (2) Carry out double digestion of pUC-Anti-EGFRvIII CAR, use agar electrophoresis to cut out the agar part of the Anti-EGFRvIII CAR DNA fragment, use the DNA extraction kit sol solution to treat, pass through the DF column and discard the filtrate, rinse the DF column, empty Centrifuge and elute the DF column, collect the centrifugate, and obtain the purified Anti-EGFRvIII CAR DNA fragment, which is the anti-EGFRvIII safe chimeric antigen receptor.
[0049] In ...
Embodiment 3
[0055] To prepare a plasmid containing the Anti-EGFRvIII CAR DNA fragment, the purified Anti-EGFRvIII CAR DNA fragment and the linearized pLent-C-GFP DNA fragment were ligated overnight at 16°C to form a pLent-Anti-EGFRvIIICAR plasmid, including the following steps:
[0056] According to the guide CD8leader nucleic acid artificial sequence, EGFRvIII scFv nucleic acid artificial sequence, CD8α nucleic acid artificial sequence, NKG2D nucleic acid artificial sequence, 2B4 nucleic acid artificial sequence, CD3ζ nucleic acid artificial sequence, self-cleaving polypeptide T2A nucleic acid artificial sequence, RQR8 nucleic acid artificial sequence, entrusted Sangon Bioengineering (Shanghai) Co., Ltd. synthesized the entire expression cassette and inserted it into the standard vector pUC, so it was named pUC-Anti-EGFRvIII CAR,
[0057] At the same time, the pUC-Anti-EGFRvIII CAR and pLent-C-GFP vectors were digested with Fast Digest AsiSI (purchased from ThermoFisher) and Fast Digest N...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com