A strain of Escherichia coli recombinant strain heterologously expressing heat-resistant nitrile hydratase and its application
A technology of nitrile hydratase and heterologous expression, applied in the field of bioengineering, can solve the problems of difficult product purification, low production efficiency, long growth cycle, etc., and achieve the effects of simplifying separation and purification steps, high production efficiency, and short fermentation cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Construction of recombinant Escherichia coli BL21 / pET24a-Cal.tNHase
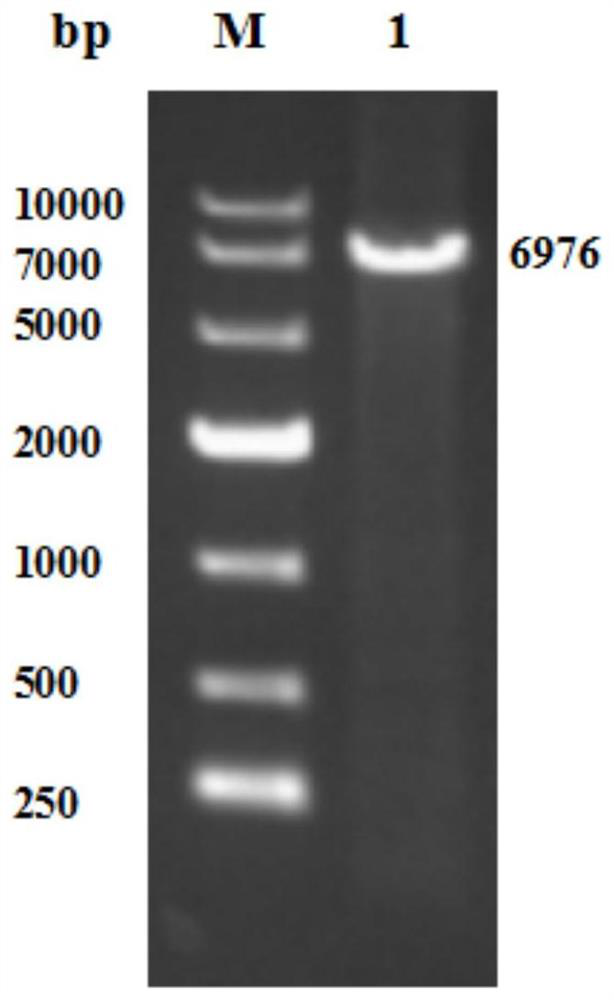
[0036] The NHase gene sequence derived from Caldalkalibacillus thermarum TA2.A1 was retrieved through NCBI, optimized through the codon online optimization website Graphical Codon Usage Analyzer, and a spacer sequence was added between the genes encoding each subunit, specifically: the encoded amino acid sequence The gene of SEQ ID NO.1, the spacer sequence shown in SEQ ID NO.4, the gene encoding the amino acid sequence SEQ ID NO.2, the spacer sequence shown in SEQ ID NO.4 and the gene encoding the amino acid sequence SEQ ID NO.3 The genes are connected sequentially to obtain the gene NHase of nitrile hydratase, the nucleotide sequence is shown in SEQID NO.5, and the electrophoresis result is shown in figure 1 Shown, synthesized by Jinweizhi Biotechnology Company.
[0037] The optimized NHase sequence does not contain restriction endonuclease sites. Specific primers P1 and P2 are designe...
Embodiment 2
[0042] The expression of embodiment 2 nitrile hydratase
[0043] Inoculate recombinant Escherichia coli BL21 / pET24-Cal.t NHase in 5 mL of LB medium with a kanamycin concentration of 100 μg / mL, and culture overnight at 37°C with shaking at 200 r / min. Inoculate the above-mentioned overnight culture into LB medium containing 100 μg / mL of kanamycin at an inoculum size of 1%, and culture it with shaking at 37°C and 200 r / min until the bacterial liquid OD 600 to 0.6-0.8, add IPTG to a final concentration of 0.4mmol / L, induce culture at 20°C for 16-20h, collect the bacteria and ultrasonically disrupt them, and analyze and identify the expression level of nitrile hydratase recombinant protein by Tris-tricine SDS-PAGE method, the results are as follows image 3 shown. After sonication and centrifugation at 12000rpm for 60min, the protein was purified with affinity chromatography column Strep Trap FF, and the specific enzyme activity of the pure recombinant nitrile hydratase was 397.35...
Embodiment 3
[0044] Embodiment 3 Cal.t NHase thermal stability analysis
[0045] The pure enzyme with a concentration of 0.5 mg / mL was treated at 60°C, and samples were taken to determine the residual enzyme activity. The enzyme activity at time 0 is defined as 100%. Such as Figure 4 As shown, 60% of the enzyme activity can still be maintained at 60°C for 6 hours. The most commonly used nitrile hydratase in industry is H-NHase derived from Rhodococcus rhodochrous J1, which has a half-life of 1 hour at 60°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com