Method for synthesizing aromatic hydrocarbon/alkane 2,2,2-trifluoroethyl selenium ether through copper catalysis and application of aromatic hydrocarbon/alkane 2,2,2-trifluoroethyl selenium ether to insecticides
A technology for the synthesis of trifluoroethylselenide and aromatic hydrocarbons, which is applied in the fields of pesticides, applications, biocides, etc., can solve the problems of less research on compounds containing 2,2,2-trifluoroethylselenide, and achieve adaptability Good performance, good activity, easy reaction and operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
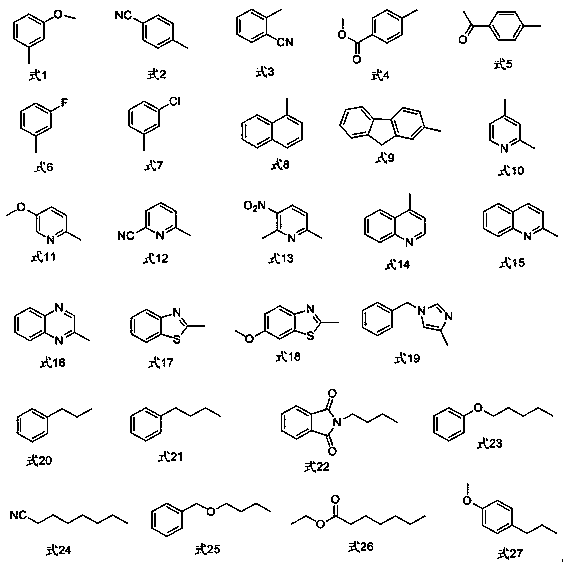
[0019] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor, then add 0.050 mmol cuprous iodide, 0.050 mmol 1,10-phenanthroline, 0.50 mmol 4-iodobenzoic acid methyl ester, 0.50 mmol of selenium powder, 1.0 mmol of 2,2,2-trifluoroiodoethane, 0.50 mmol of sodium borohydride, and finally 5 mL of N,N-dimethylformamide was added, and the reaction was stirred at 95°C for 16 h in a closed system. Cooled to room temperature, extracted 3 times with dichloromethane, 15 mL each time, combined the organic phases, washed 3 times with distilled water, dried the organic phase with anhydrous magnesium sulfate, filtered, and then rotary evaporated to remove the organic solvent; the obtained crude product was passed through Silica gel column chromatography, eluting with n-pentane, gave methyl 4-(2,2,2-trifluoroethylselenyl)benzoate (isolation yield 68%). 1 H NMR (400 MHz, CDCl 3 ) δ 7.81 (dd, J = 132.4, 7.3 Hz, 4H), 3.94 (s, 3H), 3.46 (q, J = 10.3 Hz, 2H). 19 F NMR ...
Embodiment 2
[0021] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor, then add 0.050 mmol cuprous iodide, 0.050 mmol 1,10-phenanthroline, 0.50 mmol 2-cyanoiodobenzene, 0.50 mmol selenium powder, 1.0 mmol 2,2,2-trifluoroiodoethane, 0.50 mmol sodium borohydride, and finally add 5 mL N,N-dimethylformamide, and stir the reaction at 95°C for 16 h in a closed system, Cooled to room temperature, extracted 3 times with dichloromethane, 15 mL each time, combined the organic phases, washed 3 times with distilled water, dried the organic phase with anhydrous magnesium sulfate, filtered, and then rotary evaporated to remove the organic solvent; the obtained crude product was passed through Silica gel column chromatography, eluting with n-pentane, gave 2-(2,2,2-trifluoroethylselenyl)benzonitrile (98% isolated yield). 1 HNMR (400 MHz, CDCl 3 ) δ 7.83 (d, J = 7.8 Hz, 1H), 7.73 (d, J = 7.6 Hz, 1H),7.56 (t, J = 7.7 Hz, 1H), 7.49 (t, J = 7.6 Hz, 1H), 3.52 (q, J = 10.2 H...
Embodiment 3
[0023] In a nitrogen atmosphere, put a polytetrafluoroethylene magnet in a reactor, then add 0.050 mmol cuprous iodide, 0.050 mmol 1,10-phenanthroline, 0.50 mmol 1-iodonaphthalene, 0.50 mmol selenium powder, 1.0 mmol 2,2,2-trifluoroiodoethane, 0.50 mmol sodium borohydride, and finally add 5 mL N,N-dimethylformamide, stir and react in a closed system at 95°C for 16 h, then cool to At room temperature, extract 3 times with dichloromethane, 15 mL each time, combine the organic phases, wash 3 times with distilled water, dry the organic phase with anhydrous magnesium sulfate, filter, and then rotary evaporate to remove the organic solvent; the obtained crude product is passed through a silica gel column Chromatography, using n-pentane as the eluent to obtain 1-(2,2,2-trifluoroethyl) naphthalene selenide (isolated yield 91%). 1 H NMR (400 MHz, CDCl 3 ) δ 8.49 (d, J = 8.4 Hz, 1H), 8.03 (d, J = 7.1 Hz, 1H), 7.92(t, 2H), 7.62 (dt, J = 27.4, 7.4 Hz, 2H), 7.44 (t, J = 7.7 Hz, 1H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com