Hydrogen storage material decomposing and hydrogen release system
A hydrogen storage material and hydrogen desorption technology, applied in the direction of hydrogen, reversible hydrogen absorption, metal/metal oxide/metal hydroxide catalyst, etc., can solve the problems of high cost, unfavorable product recovery and reuse, and low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~4 and comparative example 1~2
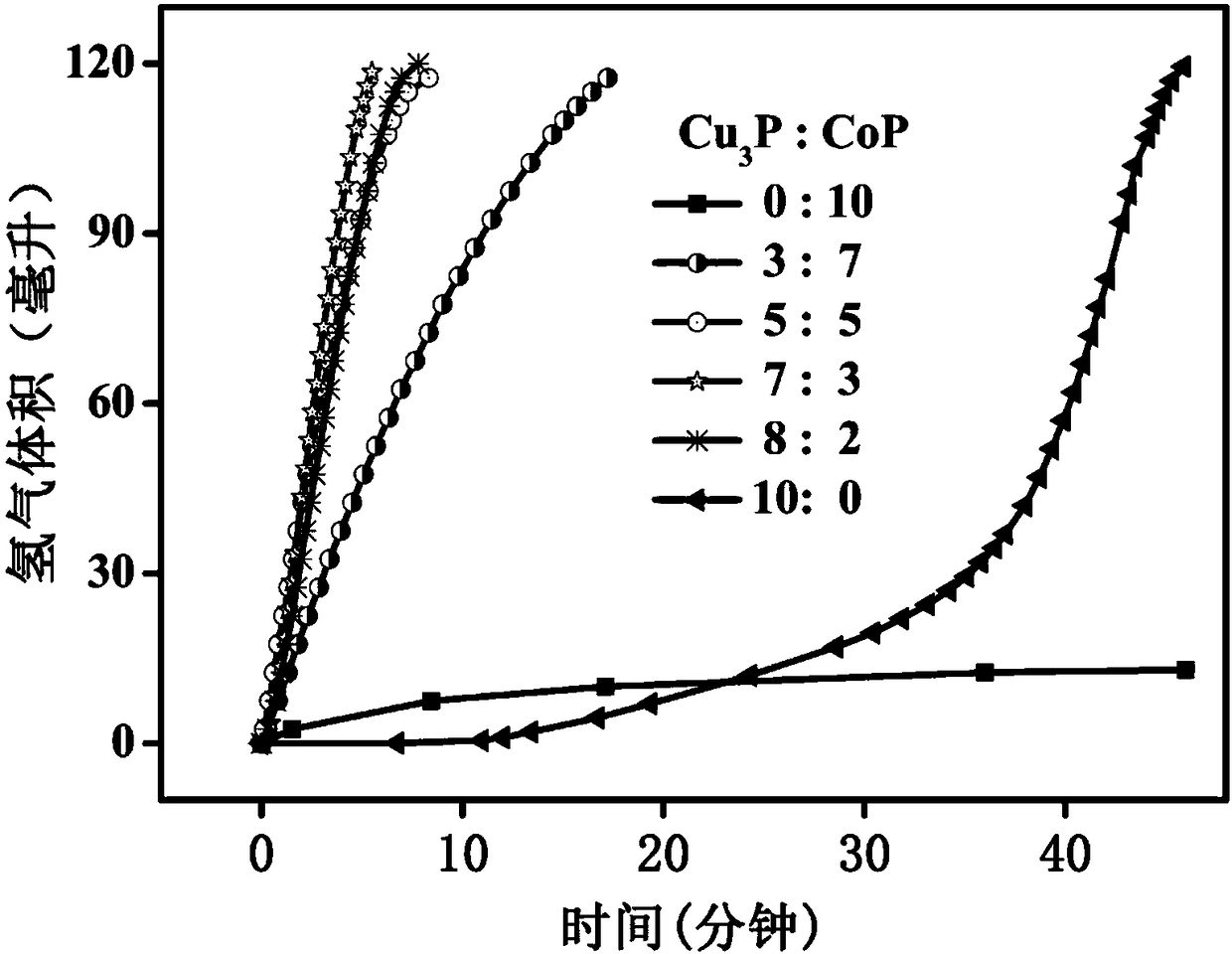
[0066] A hydrogen storage material decomposes the hydrogen release system, and measures the influence of the mass ratio of the metal compound in the catalyst on the catalytic rate, that is, changes the ratio of the metal compound in the catalyst in the system, as shown in Table 1, and calculates the hydrogen production rate of the system.
[0067] The system includes 50 mg of ammonia borane, 5 mL of a mixed solution of sodium hydroxide and methanol, 10 mg of CoP and Cu 3 P is a catalyst mixed according to different mass ratios; wherein the concentration of sodium hydroxide in the mixed solution of sodium hydroxide and methanol is 0.6mol / L;
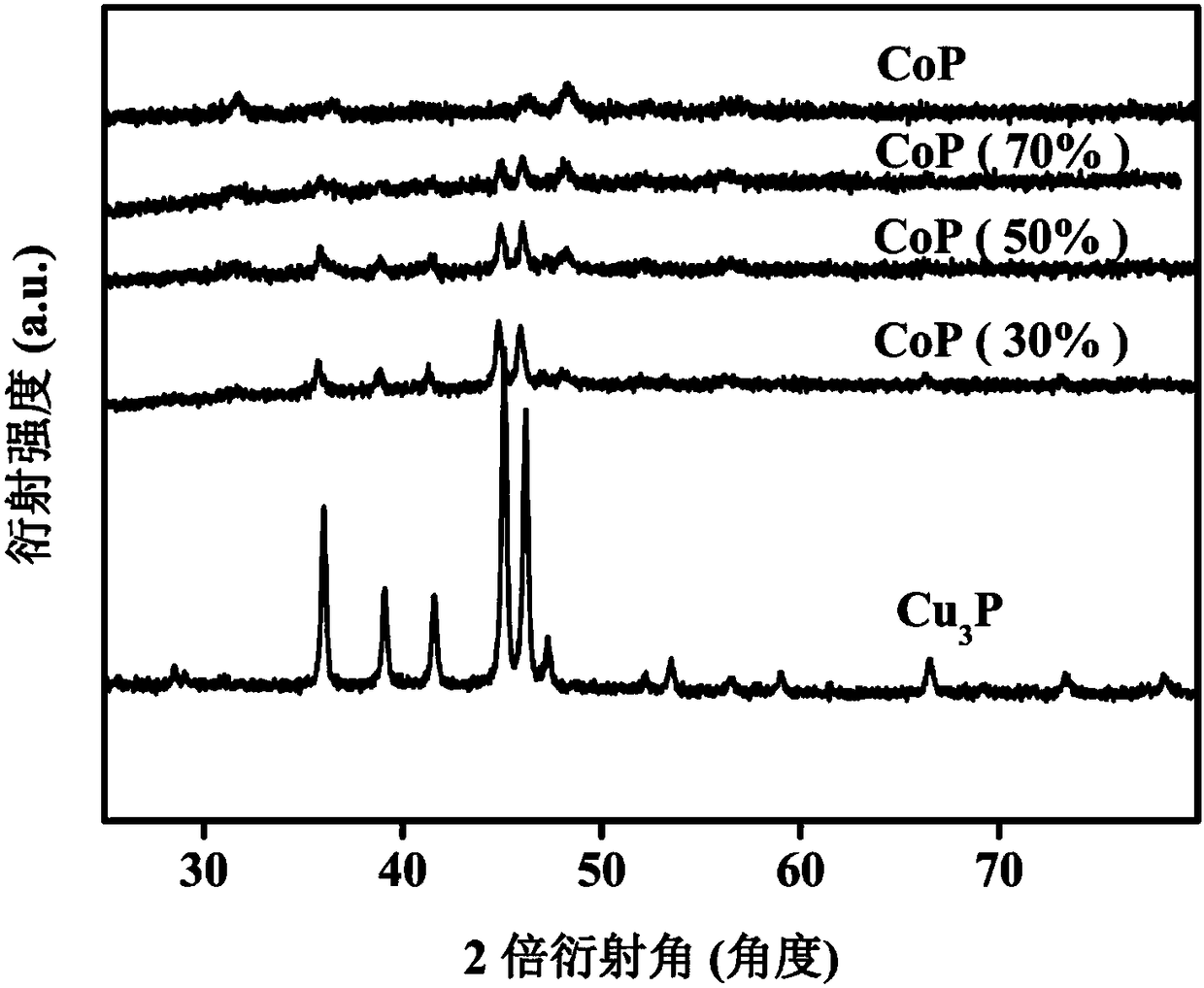
[0068] CoP and Cu 3 Preparation of catalysts mixed with P according to different ratios: different mass ratios of CoP and Cu 3 P was mixed evenly in the grinding platinum, and then each sample was fully ground, and after fully grinding, the catalyst was obtained. Do powder diffraction to the obtained catalyst, the result obtained is as f...
Embodiment 5~10
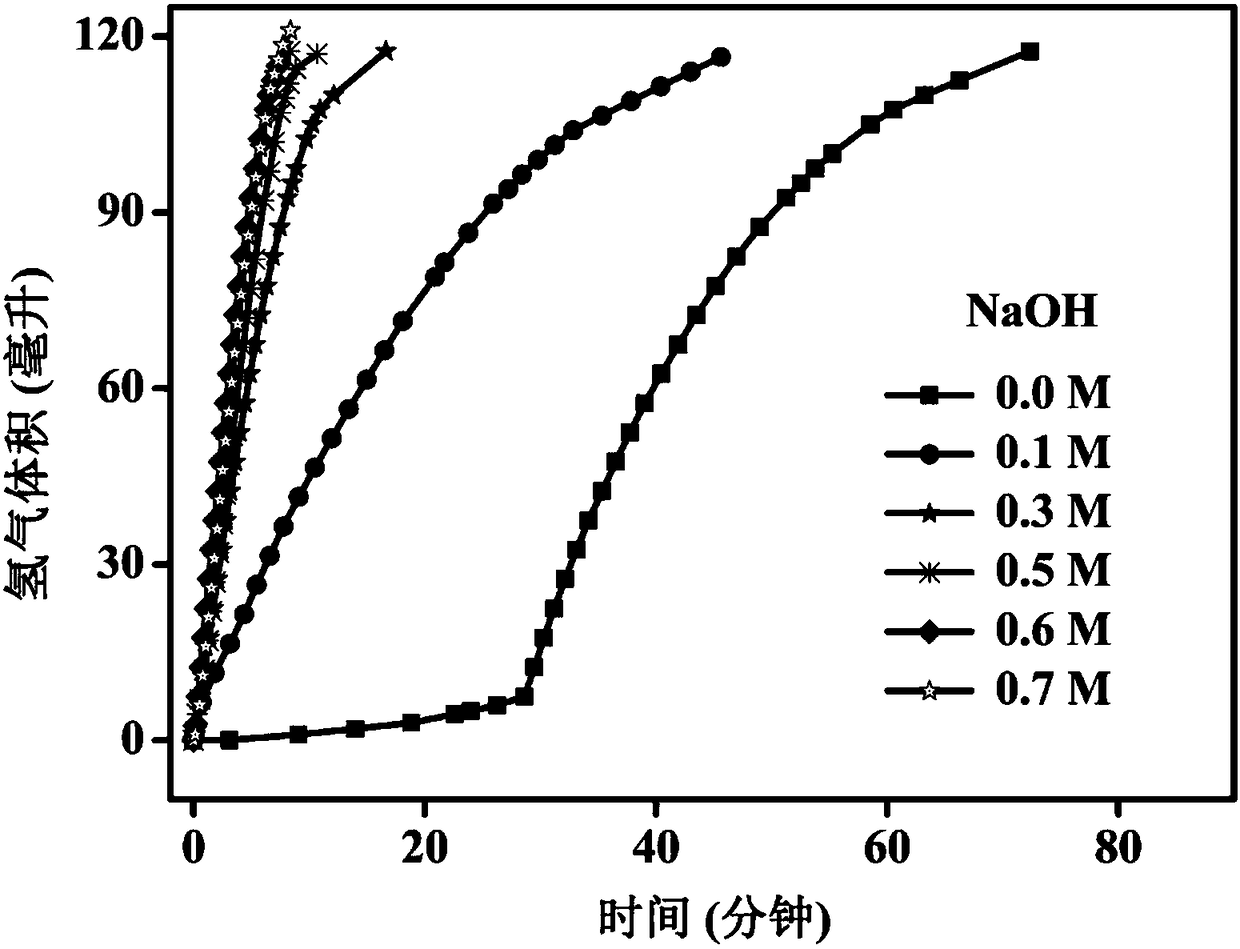
[0074] A hydrogen storage material decomposes the hydrogen release system, and measures the influence of the amount of alkali in the system on the catalytic rate, that is, the method steps are the same as in Example 2, the only difference is that the concentration of sodium hydroxide in the system is changed, as shown in Table 2, the calculation system the rate of hydrogen production.
[0075] The hydrogen production rate obtained by different sodium hydroxide concentrations in the mixed solution of table 2 sodium hydroxide and methanol
[0076]
[0077] The hydrogen storage material is used to decompose the hydrogen release system to decompose and release hydrogen. At 298K, the catalyst is added to the reaction vessel, and then the mixed solution of sodium hydroxide and methanol dissolved in ammonia borane is injected, and the gas pipe is recorded at different times. volume of hydrogen gas. Draw a curve with each hydrogen volume versus time, such as image 3 shown. From ...
Embodiment 11~15 and comparative example 3~4
[0079] A hydrogen storage material decomposes the hydrogen release system, and measures the influence of the mass ratio of the metal compound in the catalyst on the catalytic rate, that is, changes the amount ratio of the metal compound in the catalyst of the system, as shown in Table 3, and calculates the hydrogen production rate of the system.
[0080] The system includes 50 mg of ammonia borane, 5 mL of a mixed solution of sodium hydroxide and methanol, 10 mg of Fe(OH) 3 and Cu(OH) 2 Catalysts mixed according to different mass ratios; wherein the concentration of sodium hydroxide in the mixed solution of sodium hydroxide and methanol is 0.5mol / L.
[0081] Fe(OH) 3 and Cu(OH) 2 Preparation: In a round bottom flask, add 250mg sodium citrate, 2.0g sodium hydroxide and 80mL distilled water and stir to dissolve to obtain mixed solution 1; dissolve 1g copper nitrate (or ferric nitrate) in 20mL distilled water to obtain mixed solution 2; The mixed solution 2 was slowly added dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com