Construction method for fructus evodiae medicinal material characteristic spectrum, and fructus evodiae medicinal material quality detection method
A technology of characteristic map and construction method, which is applied in the field of quality detection of Evodia rutaecarpa and construction of characteristic map of Evodia rutaecarpa, can solve the problems of inability to fully represent the characteristics of Evodia rutaecarpa, inability to identify Evodia rutaecarpa, and low resolution of chromatographic peaks. Large quantity, good separation of peaks, good processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
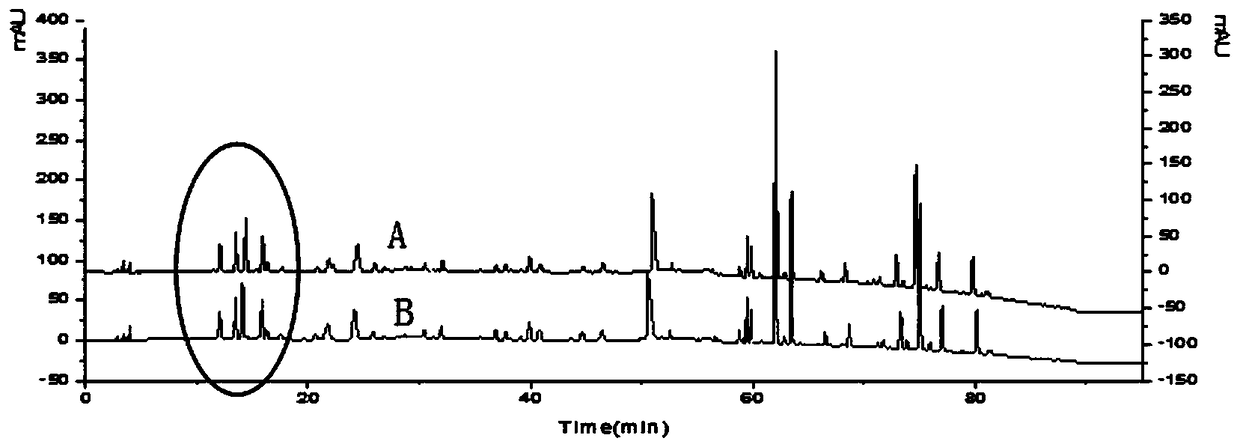
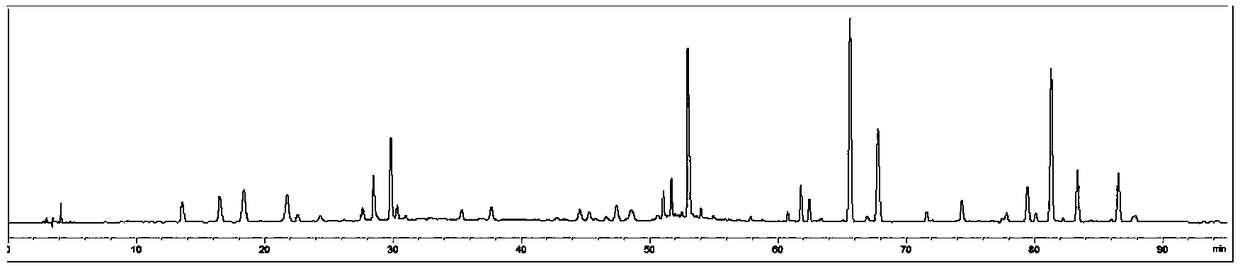
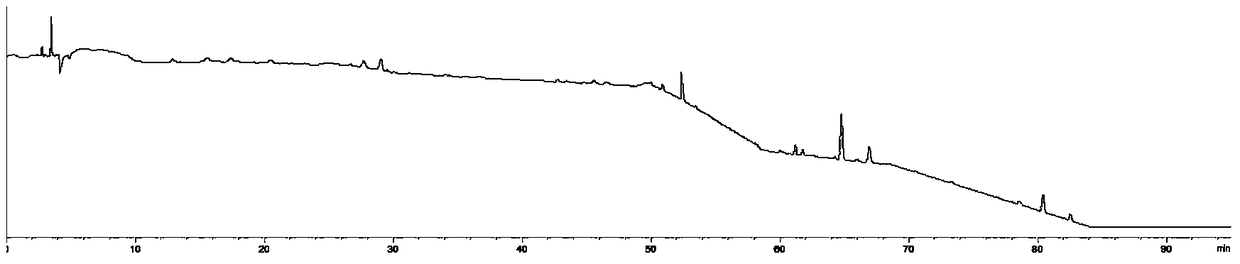
[0044] Establishment of characteristic map of Evodia rutaecarpa
[0045] In order to make the medicinal materials sufficiently representative, medicinal materials from different origins or different commodity specifications and grades were collected as test samples.
[0046] Instruments and materials
[0047] Agilent 1200 Series high-performance liquid chromatography system, AE240 electronic analytical balance (METTLERTOLEDO), KQ2200B ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.). Formic acid (analytical grade, Kemiou Chemical Reagent Co., Ltd.), acetonitrile was chromatographic grade (US TEDIA Company), water was Yibao purified water, and other reagents were analytical grade.
[0048] Choice of Chromatographic Conditions
[0049] (1) Selection of mobile phase: select acetonitrile and formic acid solution to make mobile phase, investigate the influence of acetonitrile-0.1% formic acid solution and acetonitrile-0.2% formic acid solution on chromatographic peaks, ...
Embodiment 2
[0073] The preparation method of the test solution is: take the medicinal material powder of Evodia rutaecarpa, accurately weigh, add 70% ethanol aqueous solution, 30% methanol aqueous solution, 0.5mol / L sodium chloride solution and acetone, weigh, soak , ultrasonically extracted, allowed to cool, and weighed again, with the same solvent to make up for the lost weight, shake well, filter, and take the filtrate to obtain the test solution of Evodia rutaecarpa.
[0074] Other steps are identical with embodiment 1.
Embodiment 3
[0076] Quality testing method of Evodia rutaecarpa
[0077] According to the method of step 1) to prepare the solution of the drug to be inspected by Evodia rutaecarpa, according to the method of step 3) to the solution of the drug to be inspected by Evodia rutaecarpa; to construct the characteristic map of the solution of the drug to be inspected by Evodia rutaecarpa according to the method of step 4), and combine it with the drug to be tested by Evodia rutaecarpa The feature maps were compared, and according to the comparison results, the authenticity of the Evodia rutaecarpa medicinal material to be inspected was detected.
[0078] Comparing the characteristic spectrum of 25 batches of Evodia rutaecarpa with the control characteristic spectrum of batches of Evodia rutaecarpa, the sample to be tested can detect 9 characteristic peaks, and the similarity with the control characteristic spectrum is 0.989. It is determined that the sample to be tested meets the quality requireme...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com