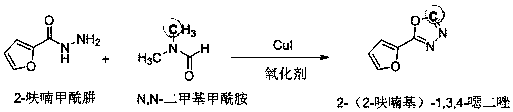
Method for building 2-(2-furyl)-1,3,4-oxadiazole in one step from DMF as carbon source
A furyl and oxadiazole technology, applied in the fields of cell biology, medicine, and materials, can solve the problems of toxic chemical reagents, and achieve the effects of easy-to-obtain reaction raw materials, mild reaction conditions, and novel synthetic routes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] In a 25 mL test tube, add 0.2 mmol of 2-furyl hydrazide, 0.05 mmol of cuprous iodide, and 0.2 mmol of potassium persulfate, add DMF (N,N-dimethylformamide) as a solvent, and dissolve at 120 Stir at ℃. After TLC (thin layer chromatography) detection, the reaction solution was cooled to room temperature, the reaction solution was filtered, extracted, the filtrate was evaporated under reduced pressure to remove the solvent, and then separated and purified by column chromatography to obtain the target product 2-(2-furyl )-1,3,4-oxadiazole, the column chromatography eluent used was a mixture of petroleum ether:ethyl acetate with a volume ratio of 3:1, and the yield was 40%.
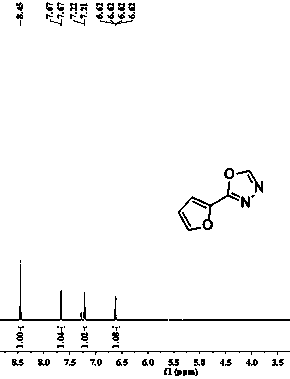
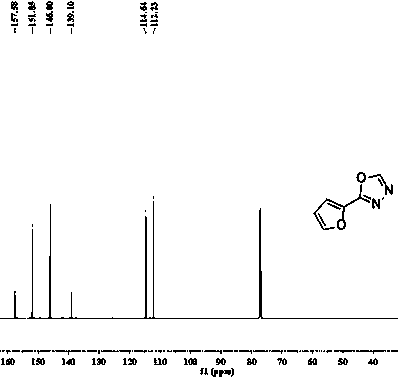
[0021] The structure of the product obtained in this embodiment is shown in figure 2 and image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com