An antibody-coupled drug targeting on EGFR, a preparation method thereof, and uses thereof
An antibody-conjugated drug, antibody technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., to achieve the effects of good repeatability, considerable yield, stable and controllable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1V
[0051] The synthesis of embodiment 1VC-MMAE (MC-VC-PAB-MMAE):
[0052] 1.1 Synthesis of MMAE
[0053] Synthetic route such as figure 1 as shown in a:
[0054] Step a: Synthesis of Boc-Dap-N-desmethylephedrine
[0055] Compound Boc-Dap-OH (500mg, 1.7mmol) and (1S, 2R)-(±)-desmethylephedrine (289.3mg, 1.9mmol) were dissolved in 10mL DMF, followed by adding DEPC (368.9mg, 2.2 mmol ) and Et 3 N (228.8mg.2.2mmol), the reaction was stirred overnight at room temperature. After the reaction was completed, add water to stop the reaction, extract three times with ethyl acetate, and extract once with saturated NaCl solution, anhydrous NaCl 2 SO 4 Let dry overnight. Separation and purification by column chromatography, the separation condition was petroleum ether: ethyl acetate = 5:1, and 485 mg of white solid was obtained. C 23 h 36 N 2 o 5 .MS(ESI)m / z:321. 1 H NMR (400MHz, DMSO-d 6 )δ7.66(d,J=8.6Hz,1H),7.36–7.25(m,4H),7.22–7.16(m,1H), 5.37(d,J=4.6Hz,1H),4.46(t,J =5.1Hz,1H...
Embodiment 2
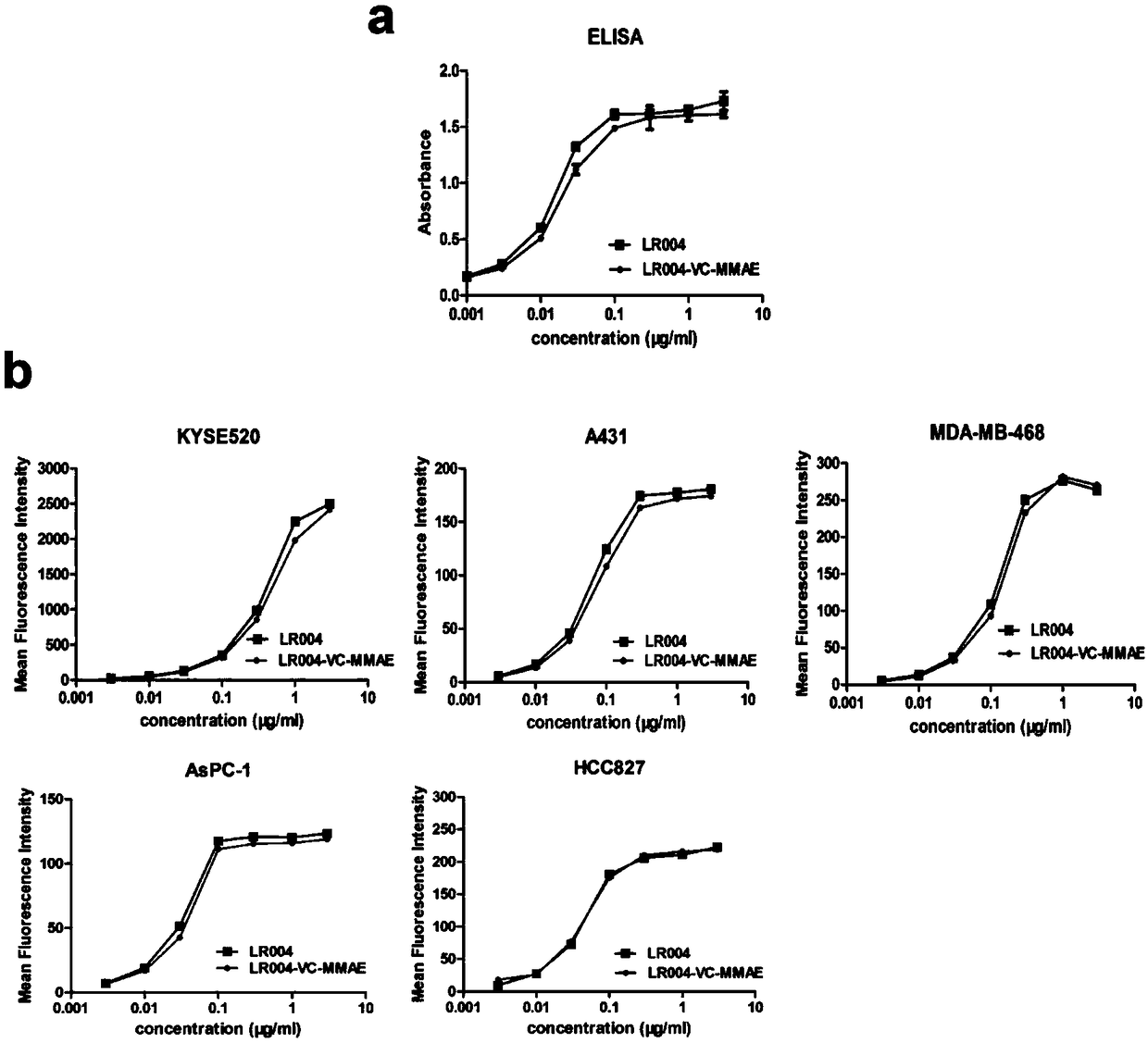
[0074] Example 2 Preparation of Antibody Drug Conjugate LR004-VC-MMAE
[0075] Include the following steps:
[0076] 1) The MC-VC-PAB-MMAE prepared in Example 1 (that is, the linker-cytotoxic drug) was dissolved in dimethyl sulfoxide to obtain a linker-cytotoxic drug stock solution.
[0077] 2) TCEP reducing agent is dissolved in buffer solution PBS (containing 1mM DTPA), and a certain molar amount of reducing agent stock solution is prepared.
[0078] 3) Replace the buffer of LR004 antibody before reduction, and the buffer system is PBS (containing 1mM DTPA). The replacement method is to replace the desalting column. The replacement method is as follows: load 2.5 mL on the desalting column, elute 3.5 mL with replacement buffer, concentrate the concentration by ultrafiltration, ultrafiltration speed 4000 rpm, and centrifuge at 4 ° C for 10 min. Configure to obtain 5mg / mL LR004 antibody solution.
[0079] 4) Mix the 5 mg / mL LR004 antibody solution in step 3) with the reduci...
Embodiment 3
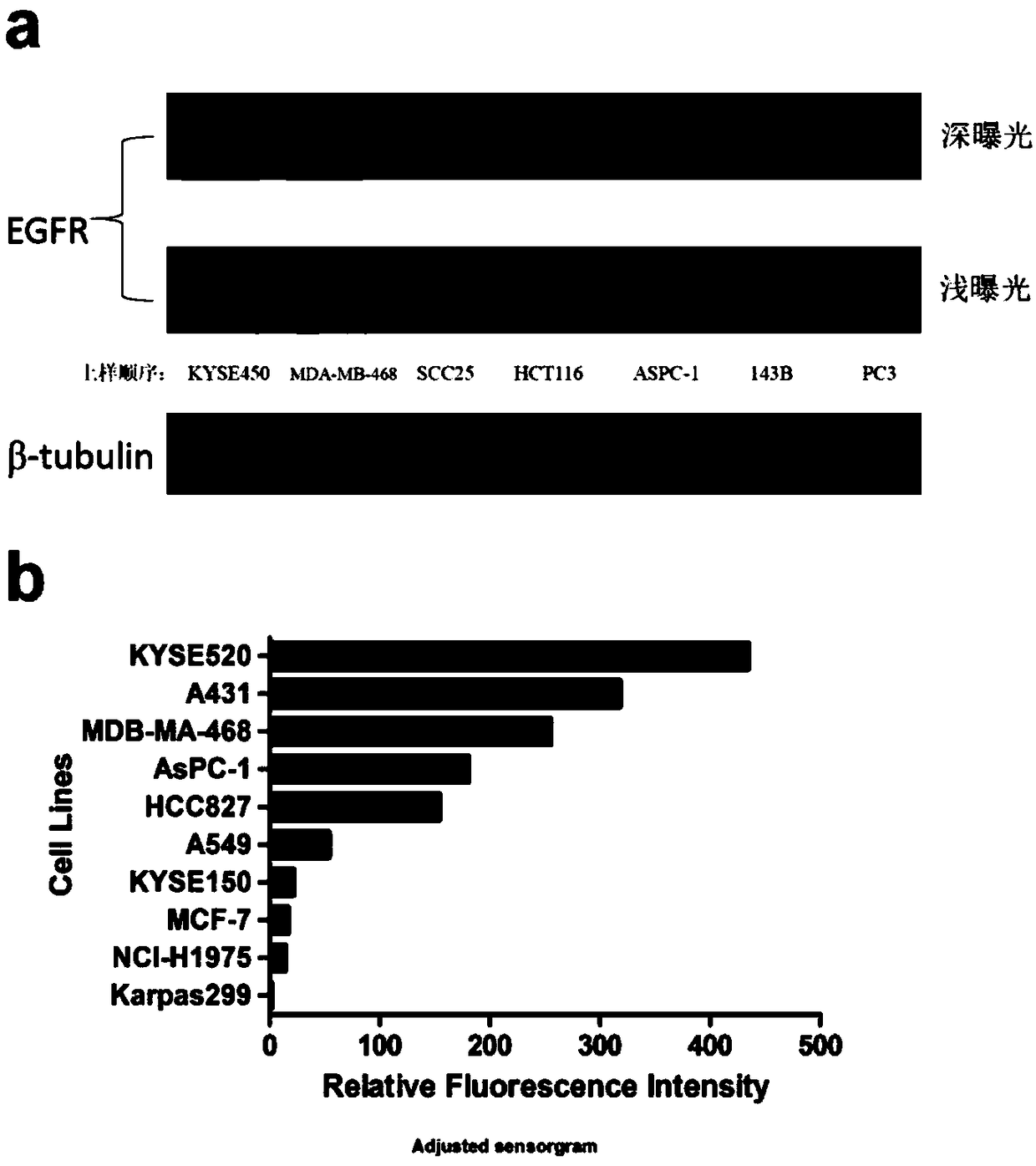
[0085] Example 3 Detection of EGFR expression in different tumor cells
[0086] 3.1 Western Blot detection of EGFR expression levels in different tumor cells
[0087] Collect logarithmic phase esophageal cancer cells (KYSE450), breast cancer cells (MDA-MB-468), head and neck cancer cells (SCC-25), colon cancer cells (HCT-116), pancreatic cancer cells (AsPC-1), Osteosarcoma cells (143B) and prostate cancer cells (PC-3). Cells were digested, collected and counted, collecting 1×10 7 The above cells were centrifuged and the supernatant was discarded for later use. Pre-cool RIPA protein extraction reagent, add protease inhibitor (phosphorylated protein needs to add phosphatase inhibitor at the same time). Add 0.1M PMSF stock solution before the start of protein extraction, and the final concentration of PMSF is 1mM. For cell counting, the number of cells is 1 x 10 7 Add 1ml of lysate, centrifuge at 4°C, store, and measure protein concentration by BCA method. The Western Blot ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com