Spherical electrocatalytic material and preparation method thereof
An electrocatalytic material, spherical technology, applied in chemical instruments and methods, physical/chemical process catalysts, inorganic chemistry, etc., can solve the problems of complex methods and high processing temperature, and achieve accelerated transmission rate, large specific surface area, good electrocatalysis performance effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
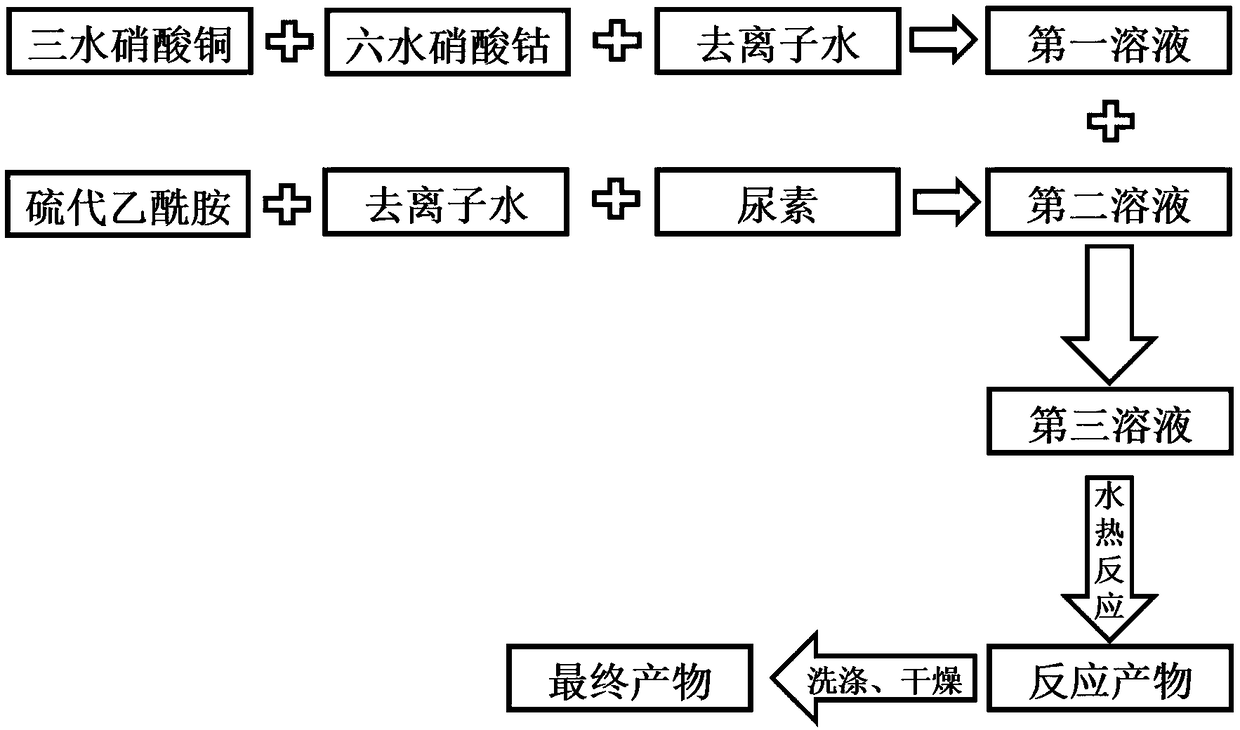
[0050] The preparation method of the spherical electrocatalytic material of the present invention comprises mixing copper nitrate, cobalt nitrate, thioacetamide, urea and water, and reacting at 190-200°C for 17-19 hours to obtain a spherical electrocatalytic material; wherein, nitric acid Copper, cobalt nitrate, thioacetamide and urea are taken according to the molar ratio of copper, cobalt, sulfur and urea in the range of 0.5-1:1-2:2-8:1-3.
[0051] Among them, the addition of urea in the reaction is to promote the spherical CuCo 2 S 4 Synthesis, so that the raw materials can be fully reacted in the subsequent hydrothermal reaction process. The sulfur source that the present invention selects is thioacetamide, does not use common sulfur sources such as thiourea, sulfur powder, sodium sulfide, and its reason is: the melting point of thioacetamide is lower than thiourea, is easy to reaction, and the product made by using thiourea has a large amount of impurities; and sulfur p...
Embodiment 1
[0068] a, according to the molar ratio of copper, cobalt, sulfur and urea is 1:2:4.35:2.1 to get raw materials: copper nitrate trihydrate 0.23g, cobalt nitrate hexahydrate 0.56g, thioacetamide 0.31g, urea 0.12g;
[0069] B, copper nitrate trihydrate and cobalt nitrate hexahydrate are dissolved in 20g of water to obtain the first solution;
[0070] c, thioacetamide is dissolved in 20g deionized water, then add urea, obtain the second solution;
[0071] d. Pour the first solution into the second solution, and mix to obtain the third solution; carry out stirring, and then carry out hydrothermal reaction; the reaction temperature of the hydrothermal reaction is controlled at 190° C., and the reaction time is controlled at 18 hours.
[0072] e. The product after the hydrothermal reaction is washed and dried sequentially to obtain a spherical electrocatalytic material S1.
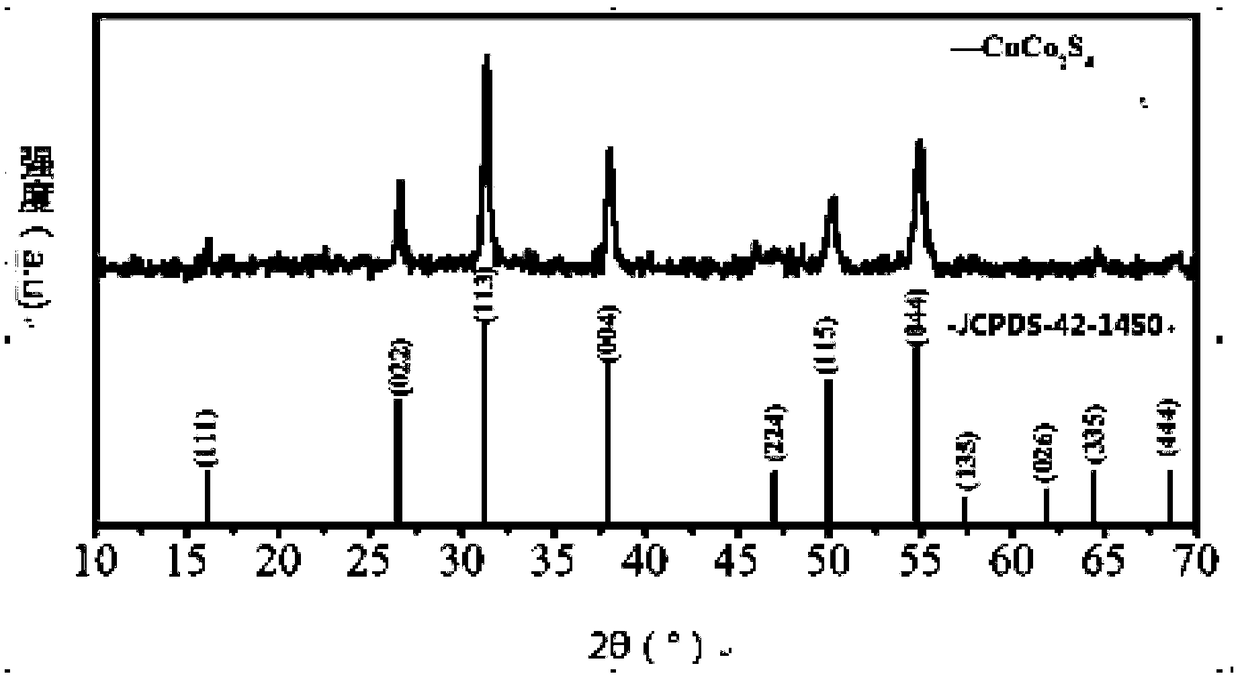
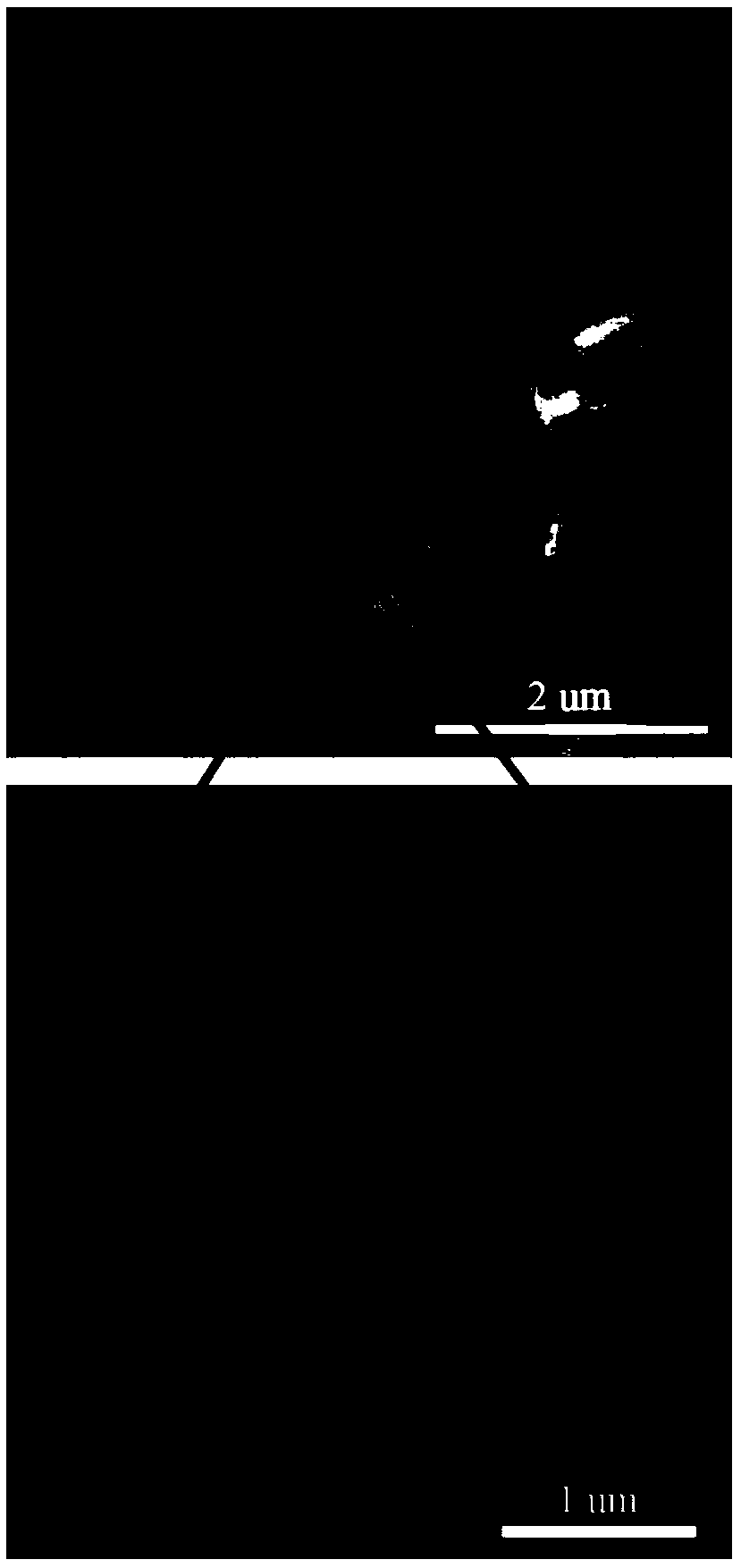
[0073] The XRD pattern of S1 is shown in Picture 1-1 , SEM image see Figure 1-2 , see the BET chart Fig...
Embodiment 2
[0075] On the basis of Example 1, the dosages of copper nitrate trihydrate, cobalt nitrate hexahydrate, thioacetamide, and urea were changed, and the temperature and reaction time of the hydrothermal reaction were changed to obtain spherical electrocatalytic materials S2-S3. For specific parameters, see Table 1.
[0076] The XRD pattern of S2 material is as follows Figure 2-1-1 As shown, the SEM image is shown in Figure 2-1-2 Shown; The XRD pattern of S3 material is as Figure 2-2-1 As shown, the SEM image is shown in Figure 2-2-2 shown. It can be seen from the figure that S2~S3 can prepare spherical CuCo 2 S 4 Material, the main component of the material is CuCo 2 S 4 , but contains impurities, and some of them are in poor shape.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com