Antimicrobial peptide with complex biological activity and preparation method and application thereof
A technology of antimicrobial peptides and bacteriostatic agents, applied in the field of antimicrobial peptides and their preparation, can solve problems such as economic losses in the aquaculture industry, and achieve the effects of good agglutinating bacteria, inhibiting fungi, and good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
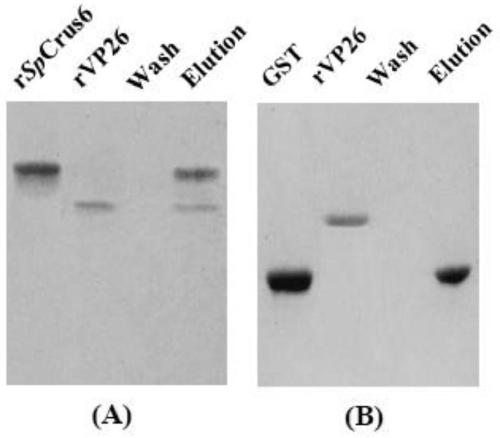
[0025] Example 1 Cloning, recombinant expression and purification of antimicrobial peptide gene Sp-crustin 6 of Scylla pseudocaveus
[0026] The amino acid sequence of the antimicrobial peptide Sp-Crustin 6 with compound biological activity of the present invention is shown in SEQ ID NO.1; the nucleotide sequence encoding the antibacterial peptide Sp-Crustin 6 is shown in SEQ ID NO.2. Antimicrobial peptide Sp-Crustin 6 was recombinantly expressed and purified according to the following steps.
[0027] (1) Use the animal total RNA extraction kit to extract the total RNA of the mixed tissue samples of 5 kinds of quality tissues such as muscle, hepatopancreas, gills, intestines, and eye stalks of the Scylla pseudocarpus, and obtain the total RNA samples of the mixed tissue of the Scylla pseudocarpus .
[0028] (2) Using the total RNA sample obtained in step (1) as a template, and using the specific primer 5'-CTGGGAAGAAATGACATG-3' as the post-primer of the reverse transcription s...
Embodiment 2
[0039] Example 2 Bacterial Binding Effect of Recombinant Scylla pseudophylla Antimicrobial Peptide rSp-Crustin 6
[0040] The concentration obtained in Example 1 was 200 μg·mL -1 The recombinant protein rSp-Crustin 6 solution was used for the binding experiment of common pathogenic bacteria. First, 200 μL of overnight cultured bacterial liquid (respectively Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, Vibrio parahaemolyticus, algae Vibrio, Vibrio harveii, Escherichia coli, Candida albicans, Pichia pastoris), with 200μL concentration of 200μg·mL -1 The target recombinant protein solution was incubated at 37°C and 180rpm for 1.5h on a shaking table in the dark, then centrifuged at room temperature at 6000rpm to collect the bacteria, washed 3 times with 1×PBS buffer for 5min each time, and then Wash the cells with 7% SDS solution for 5 minutes, then wash the cell pellet with 500 μL 1×PBS buffer once, centrifuge at room temperature at 6000 rpm to collect the cel...
Embodiment 3
[0042] Example 3 The liquid bacteriostasis of the recombined Scylla pseudophylla antimicrobial peptide rSp-Crustin 6
[0043] The concentration obtained in Example 1 was 200 μg·mL -1 The purpose of the recombinant protein rSp-Crustin 6 solution is to carry out the liquid antibacterial experiment of common pathogenic bacteria, first Staphylococcus aureus, Bacillus subtilis, Bacillus megaterium, Micrococcus luteus, Vibrio parahaemolyticus, Vibrio alginolyticus, Harvey Vibrio, Escherichia coli, Pseudomonas aeruginosa, Candida albicans, and Pichia pastoris were routinely cultured overnight as pathogenic bacteria. On the next day, 100 μL of recombinant Scylla syringae antimicrobial peptides diluted 2-fold with 1×PBS buffer The rSp-Crustin 6 protein solution was mixed with 100 μL of the target pathogenic bacteria solution, and placed in the wells of a clean disposable 96-well microplate for incubation. At the same time, GST protein was used as the control group, and each spotting 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com