Preparation method of modified separator for lithium ion battery
A lithium-ion battery and separator technology, applied in battery pack parts, separators/films/diaphragms/spacers, circuits, etc., can solve the problems of large pores, poor thermal stability, low surface energy and hydrophobicity, etc. Achieve the effect of wide electrochemical window, high ionic conductivity, and improved wettability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
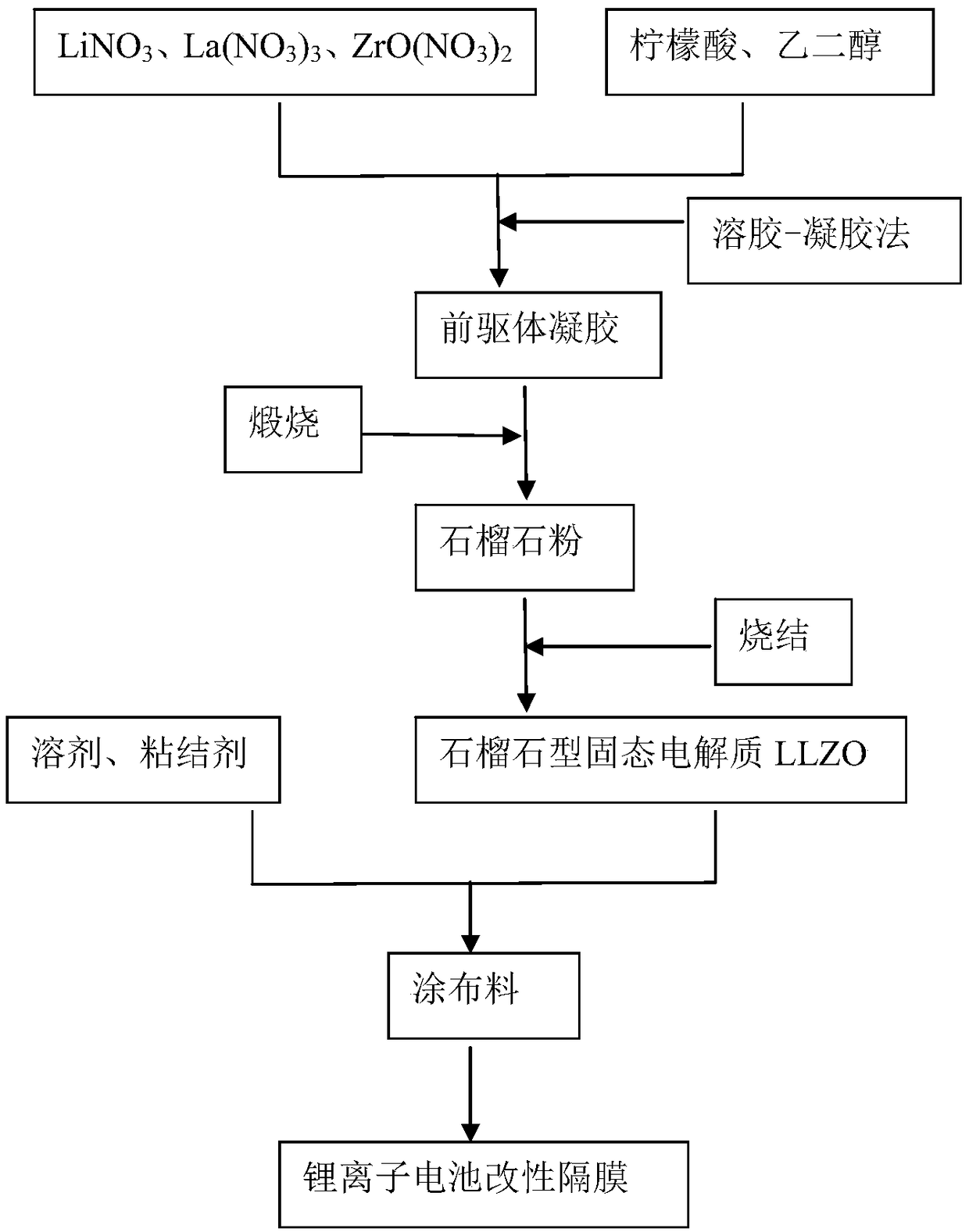
[0035] (1) Preparation of garnet-type solid electrolyte LLZO
[0036] 7.24g LiNO 3 , 19.48g La(NO 3 ) 3 , 6.94g ZrO(NO 3 ) 2 Dissolve in 300ml deionized water and mechanically stir at 100°C to completely dissolve the solid. After the solid was fully dissolved, 37.83g of citric acid and 0.36mol of ethylene glycol were added, the temperature was raised to 120°C, and mechanical stirring was continued to carry out the evaporation reaction for 12 hours. When the reaction solution was about 80ml, the reaction was stopped to obtain a precursor gel. Transfer the obtained precursor gel to a crucible, put it into a muffle furnace for calcination, and calcine at 400°C and 800°C for 5h respectively; after calcination, grind it into a powdery solid; put the powdery solid at 1050°C After sintering for 12 hours, the garnet-type solid electrolyte LLZO was obtained.
[0037] (2) Preparation of coating material
[0038] Take 0.153g garnet-type solid electrolyte LLZO as a filler, 0.153g PVD...
Embodiment 2
[0044] (1) Preparation of garnet-type solid electrolyte LLZO
[0045] 9.31g LiNO 3 , 19.48g La(NO 3 ) 3 , 6.94g ZrO(NO 3 ) 2 Dissolve in 300ml deionized water and mechanically stir at 100°C to completely dissolve the solid. After the solid was fully dissolved, 37.83g of citric acid and 0.36mol of ethylene glycol were added, the temperature was raised to 110°C, and mechanical stirring was continued for evaporating reaction for 14 hours. When the reaction solution was about 80ml, the reaction was stopped to obtain a precursor gel. Transfer the obtained precursor gel to a crucible, put it into a muffle furnace for calcination, and calcine at 350°C and 750°C for 6h respectively; after calcination, grind it into a powdery solid; put the powdery solid at 1000°C After sintering for 14 hours, the garnet-type solid electrolyte LLZO was obtained.
[0046] (2) Preparation of coating material
[0047] Take 0.32g garnet-type solid electrolyte LLZO as a filler, 0.16g PVDF as a binder...
Embodiment 3
[0051] (1) Preparation of garnet-type solid electrolyte LLZO
[0052] 9.31g LiNO 3 , 19.48g La(NO 3 ) 3 , 6.94g ZrO(NO 3 ) 2 Dissolve in 300ml deionized water and mechanically stir at 100°C to completely dissolve the solid. After the solid was fully dissolved, 37.83g of citric acid and 0.42mol of ethylene glycol were added, the temperature was raised to 130°C, and mechanical stirring was continued for 10 hours of evaporation reaction. When the reaction solution was about 80ml, the reaction was stopped to obtain a precursor gel. The resulting precursor gel was transferred to a crucible, put into a muffle furnace for calcination, and calcined at 450°C and 850°C for 4 hours respectively; after calcining, it was ground to form a powdery solid; the powdery solid was then heated at 1100°C After sintering for 10 hours, the garnet-type solid electrolyte LLZO was obtained.
[0053] (2) Preparation of coating material
[0054] Take 0.3g garnet-type solid electrolyte LLZO as a fil...
PUM
| Property | Measurement | Unit |
|---|---|---|
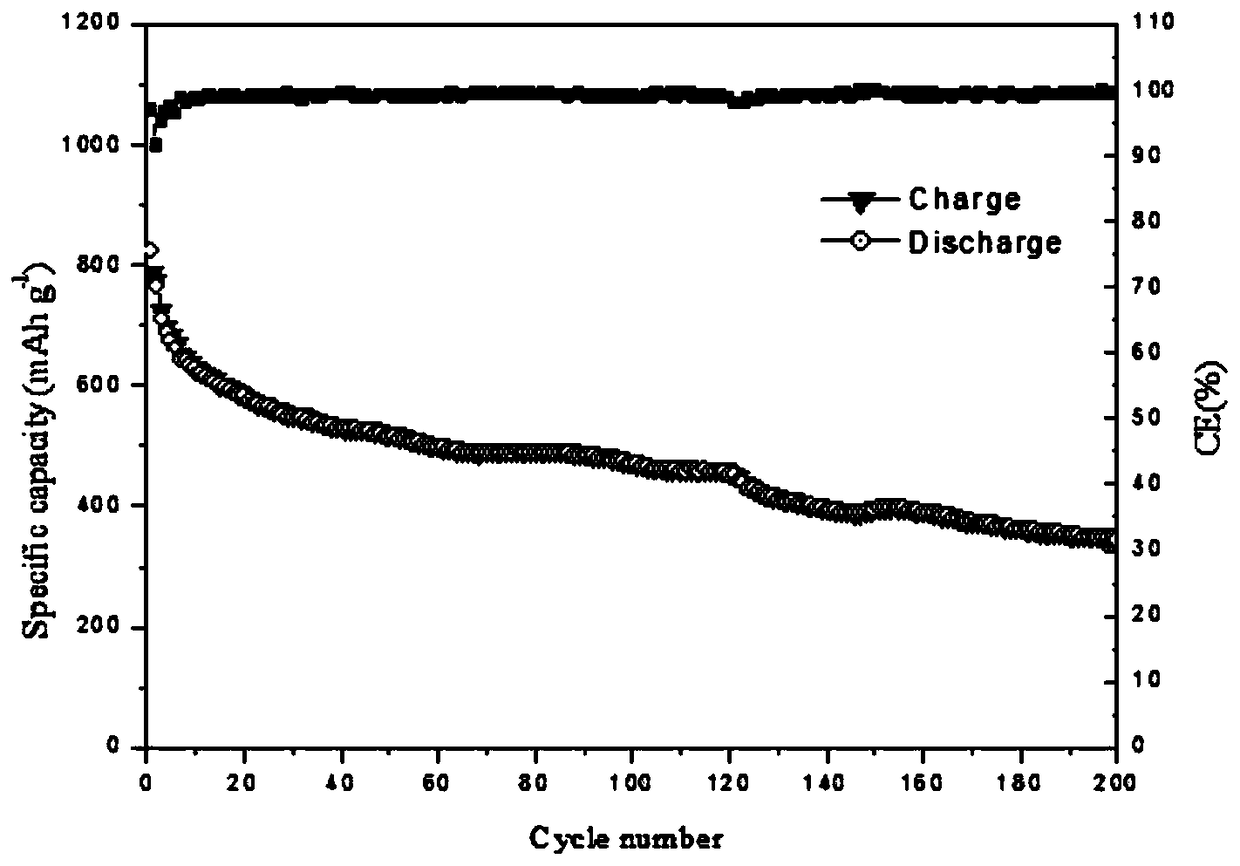
| Coulombic efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com