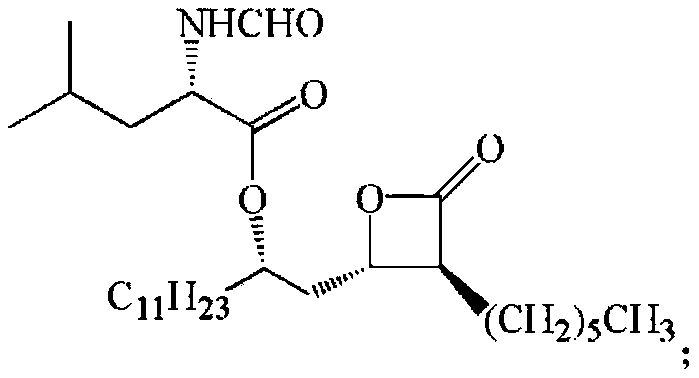
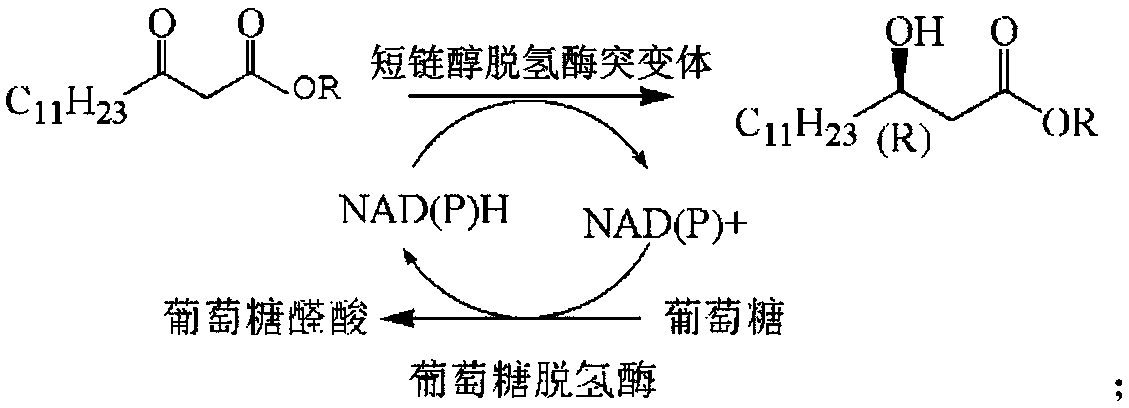
Novosphingobium short-chain alcohol dehydrogenase mutant and application thereof
A new sphingolipid and short-chain alcohol technology, applied in the direction of enzymes, oxidoreductases, enzymes, etc., can solve the problems of harsh reaction conditions and low chiral ee value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A short-chain alcohol dehydrogenase mutant from Neosphingomum marinum, which uses the amino acid sequence of NA-ADH of Neosphingomum marinum short-chain alcohol dehydrogenase as a template, and transforms the first amino acid sequence of NA-ADH according to site-directed mutagenesis technology. NA-ADH-M1 was constructed by mutating glycine at position 145 to alanine.
Embodiment 2
[0044] A short-chain alcohol dehydrogenase mutant from Neosphingomum marinum, which uses the amino acid sequence of NA-ADH of Neosphingomum marinum short-chain alcohol dehydrogenase as a template, and transforms the first amino acid sequence of NA-ADH according to site-directed mutagenesis technology. The 199-position isoleucine was mutated to leucine to construct NA-ADH-M2.
Embodiment 3
[0046] A short-chain alcohol dehydrogenase mutant from Neosphingomum marinum, which uses the amino acid sequence of NA-ADH of Neosphingomum marinum short-chain alcohol dehydrogenase as a template, and transforms the first amino acid sequence of NA-ADH according to site-directed mutagenesis technology. NA-ADH-M3 obtained by mutating glycine at position 145 to alanine and isoleucine at position 199 to leucine.
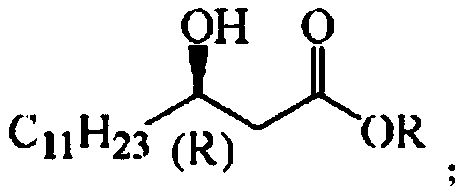
[0047] Application of the N. marine short-chain alcohol dehydrogenase mutant obtained from the above-mentioned N. marine N. sphingolis short-chain alcohol dehydrogenase NA-ADH in the preparation of (R)-beta-hydroxy myristate, wherein The structural formula of (R)-β-hydroxytetradecanoate is:
[0048]
[0049] R in the molecular structural formula represents a saturated alkyl group with 1 to 8 carbon atoms, and (R) represents chirality. Taking the preparation of (R)-β-hydroxytetradecanoic acid methyl ester and (R)-β-hydroxytetradecanoic acid ethyl ester as an example, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com