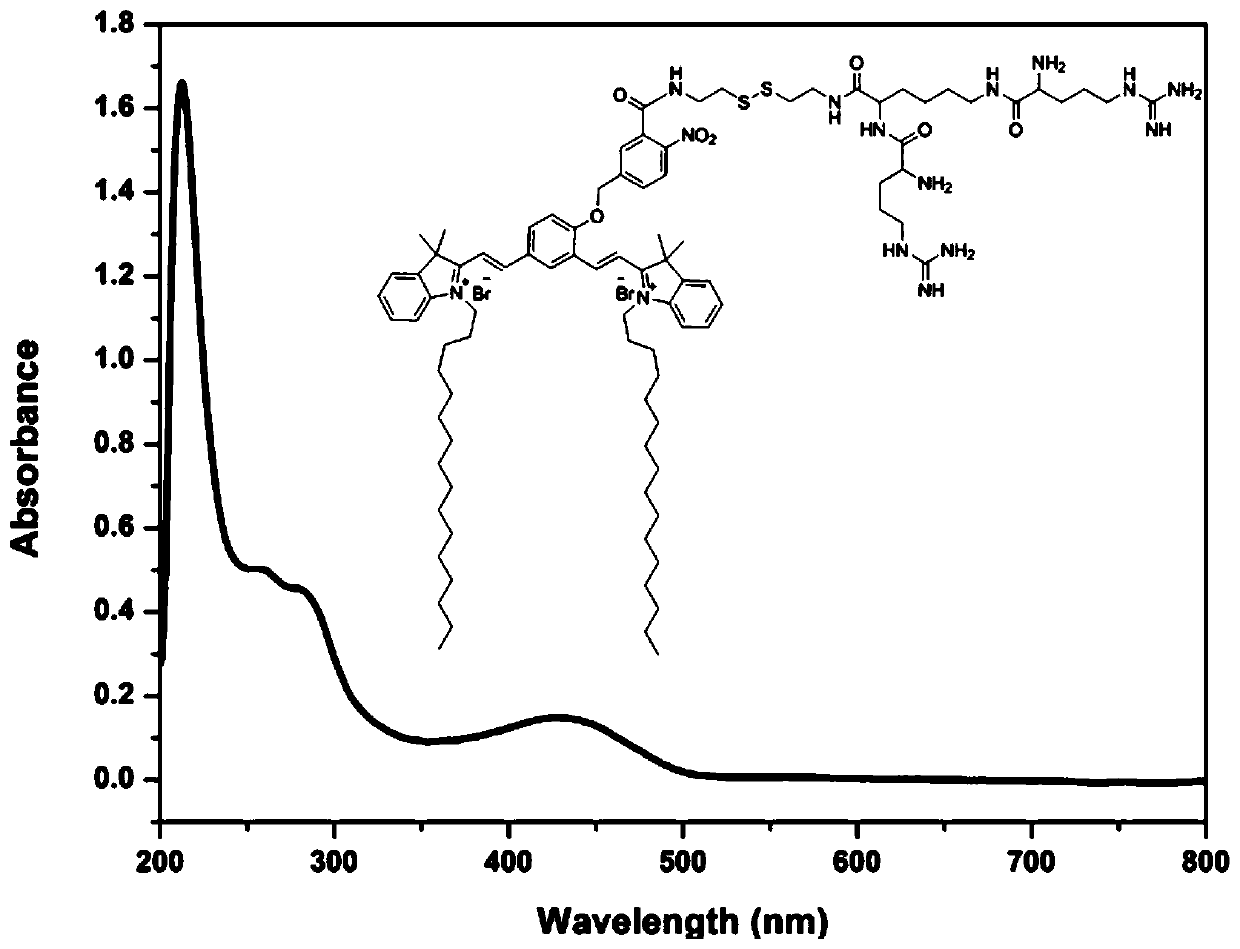
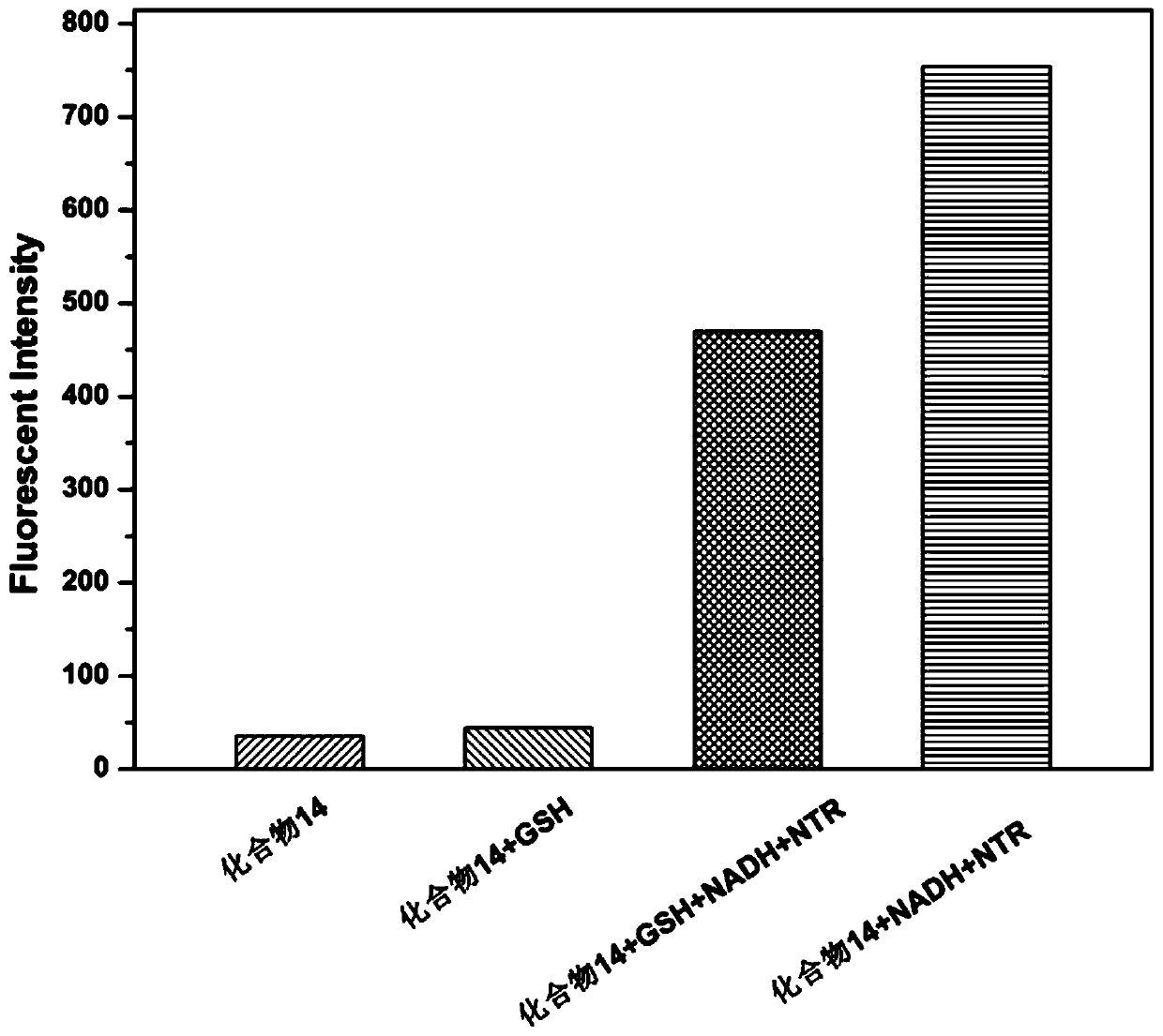
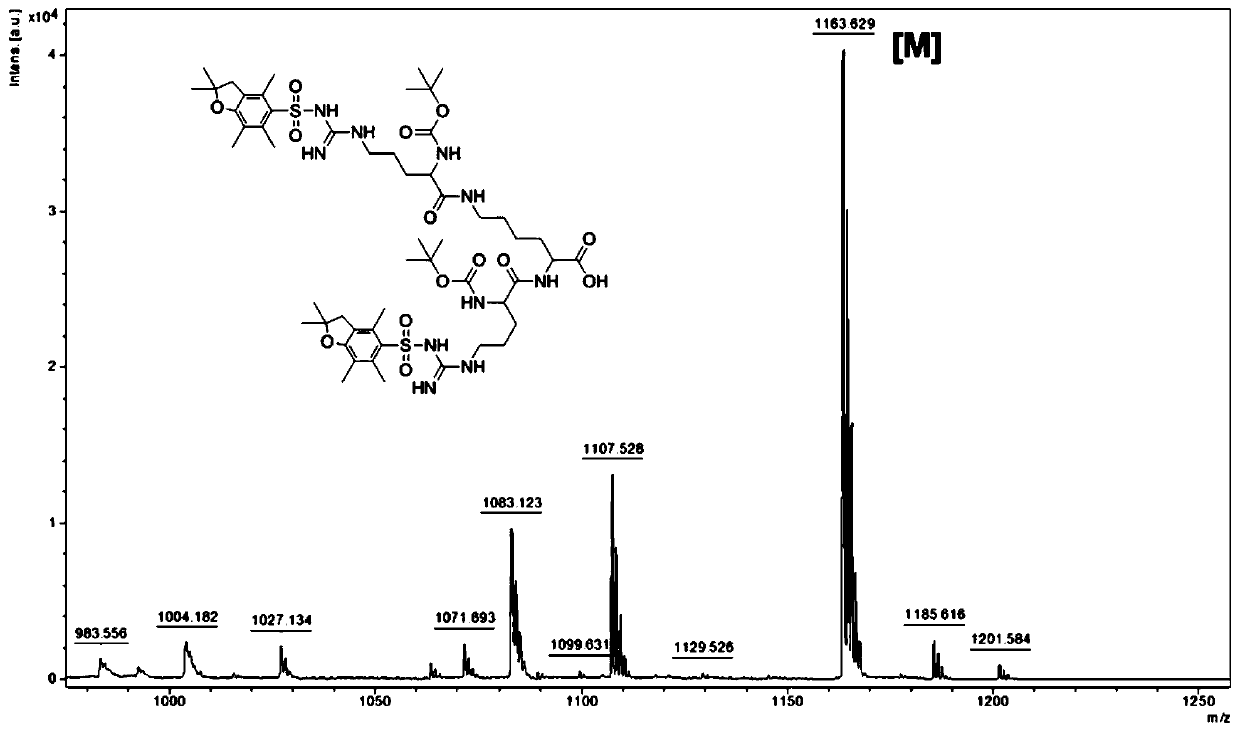
Near-infrared dyes, their targeted imaging agents, nanocarriers and anticancer drugs and their applications
A compound, CH2 technology, applied in the field of targeted diagnosis and treatment, biomedicine, can solve the problems of increasing difficulty in diagnosis, difficulty in imaging positioning, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] preparation.
[0112] d is an integer ranging from 1 to 7, and compounds corresponding to different d values are prepared by selecting different raw materials according to the method of this embodiment. Take d=4 as an example:
[0113]
[0114] Preparation of intermediate 1: Boc-Arg(Pbf)-OH 11.3g (0.02mol, 2.5eq) and benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate ( HBTU) 9.76g (0.024mol, 3eq) were dissolved in 20mL N,N-dimethylformamide (DMF), after mixing in a 250mL round bottom flask, N,N-diisopropylethylamine (DIEA ) 3.33g (0.024mol, 3eq) reacted. 2 g (0.008 mol, 1 eq) of L-lysine methyl ester hydrochloride was dissolved in 20 mL of DMF, and added dropwise to the above reaction solution. N 2 The reaction was carried out at 30° C. for 48 h under protection, and the reaction process was monitored by TLC. After the reaction, the DMF was distilled off under reduced pressure with a rotary evaporator, and 200 mL of dichloromethane was added to r...
Embodiment 2
[0118] Preparation of Linker Molecules Containing Reduction Sensitive Bonds:
[0119] Disulfide Linked Molecules:
[0120]
[0121] p and q are independently 1
[0122] Preparation of Compound 3: In a 1L round bottom flask, 8g (0.035mol, 1eq) of cystamine dihydrochloride was dissolved in 300mL of methanol, and then 7.07g (0.070mol, 2eq) of triethylamine was added. Dissolve 6.7g (0.031mol, 0.88eq) of di-tert-butyl dicarbonate in 200mL of methanol, under stirring, in an ice-water bath, slowly drop the methanol solution of di-tert-butyl dicarbonate into the methanol solution of cystamine dihydrochloride . React overnight at 30°C, then use a rotary evaporator to distill off methanol under reduced pressure, and add 100 mL of dichloromethane to redissolve. The organic phase was washed twice with 50 mL of saturated NaCl solution, dried over anhydrous sodium sulfate, filtered with suction, and spin-dried. 7.2 g of crude product was obtained by weighing, and the crude yield was ...
Embodiment 3
[0124] Preparation of near-infrared fluorescent dyes:
[0125]
[0126]
[0127] Among them, R 1 for C 2-18 Alkyl, R 2 for -C(O)R 5 , R 3 for H, R 4 for H, R 5 for NR 6 R 7 , R6 and R7 are -(CH 2 ) p -S-S-(CH 2 ) q -NR 8 R 9 , where p is 1, q is 1, R 8 and R 9 for-C(O)-CHR 10 R 11 , R 10 and R 11 is an independent Q, Q is selected from the following groups:
[0128]
[0129]
[0130] r is an integer of 1-4; k is an amino acid repeating unit, preferably the amino acid is lysine or arginine.
[0131] Compounds with different substituents are prepared by selecting the raw materials corresponding to the substituents as follows.
[0132] For example:
[0133] 3.1 Preparation of Intermediate 4:
[0134]
[0135] Mix 10g (0.082mol, 1eq) of salicylaldehyde with 4.92g (0.164mol, 2eq) of formaldehyde, and then add 100mL of concentrated hydrochloric acid dropwise to the mixture in an ice-water bath. After reacting at room temperature for 5 h, it was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com