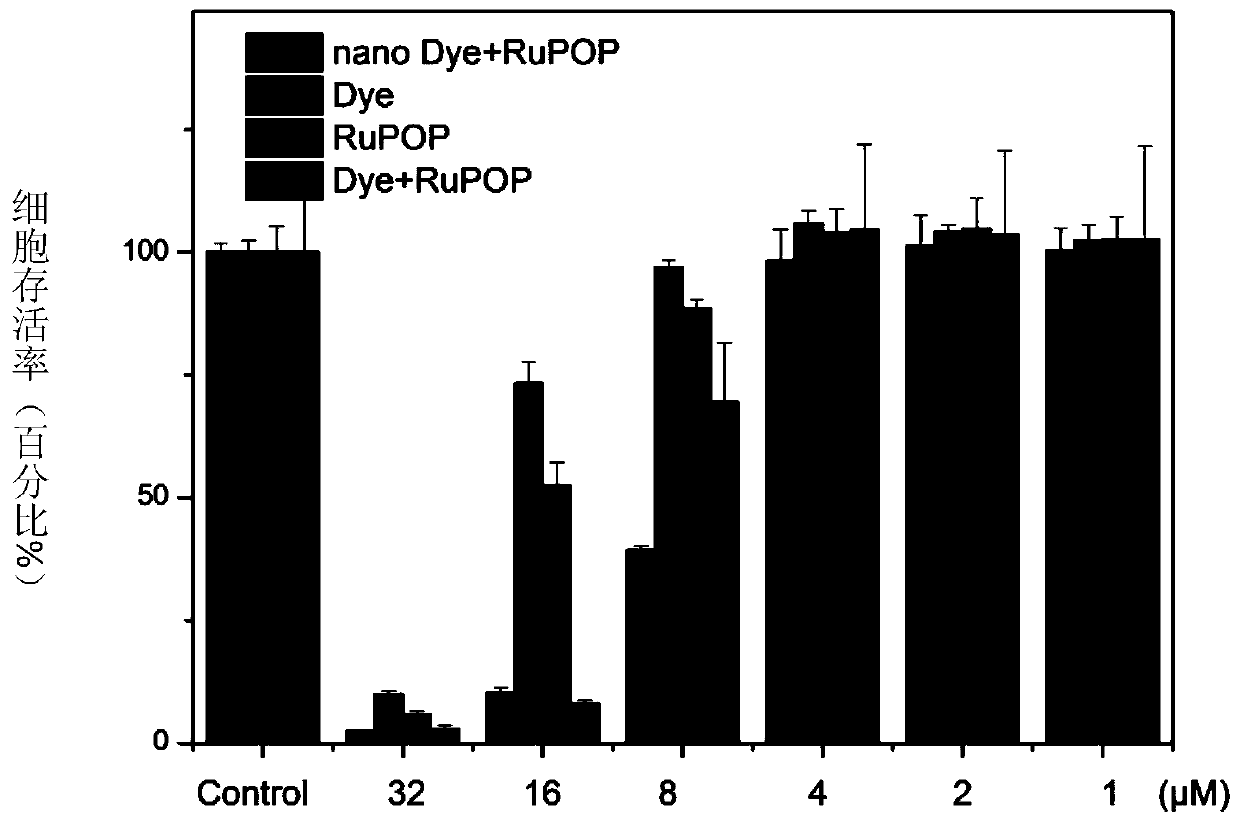
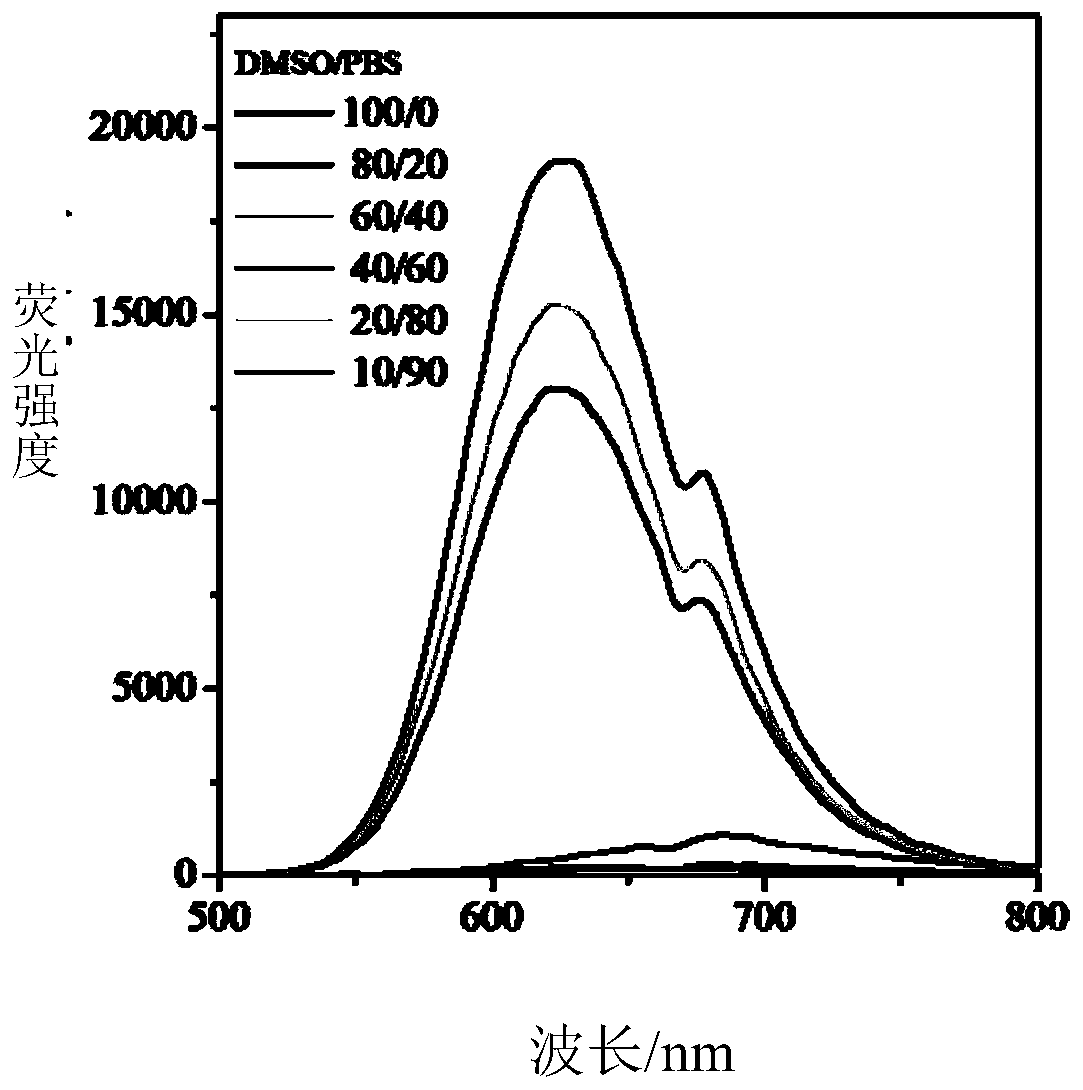
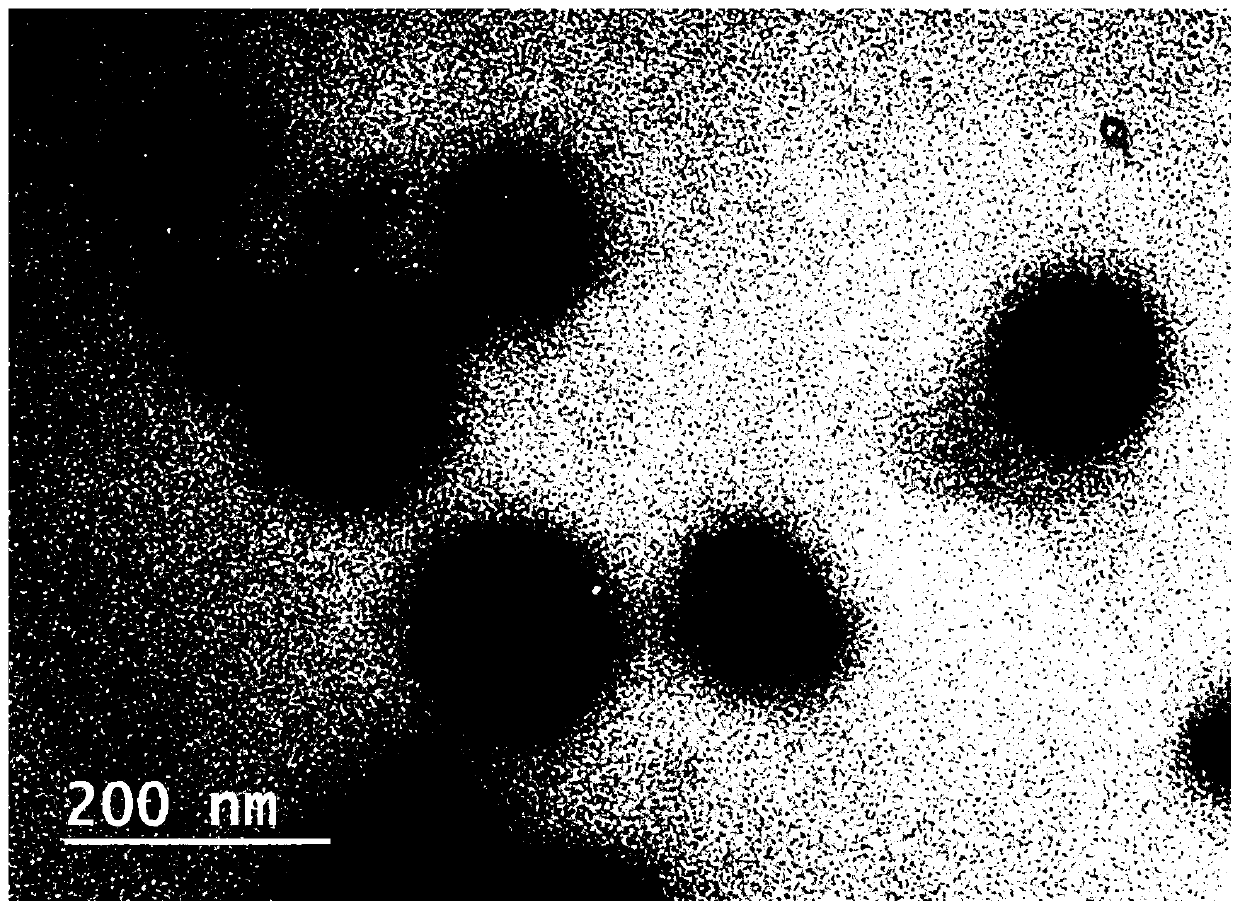
A nanomaterial with aggregation-induced luminescent effect and its application
A nanomaterial and a single technology, applied in the field of nanomaterials with aggregation-induced luminescent effect, can solve problems such as dye leakage, easy damage to dye molecular structure or fluorescent properties, to expand the synthesis scale, avoid fluorescence quenching phenomenon, and realize commercial The effects of liquefaction and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Synthesis of Compound 1A
[0031]
[0032] A solution of 4-formylphenylboronic acid (150 mg, 1.0 mmol) in THF (10 mL) was added to 4-bromo-7-[4-(diphenylamino)phenyl]-2,1,3-benzothio Oxadiazole (458 mg, 1.0 mmol) was in a mixed solution of toluene (10 mL) and 2M aqueous sodium carbonate solution (2 mL). Tetrakis(triphenylphosphine)palladium (1.73mg, 0.0015mmol) was added to the reaction at room temperature, and the temperature was raised to 100°C and refluxed overnight. The reaction solution was cooled to room temperature, most of the toluene and tetrahydrofuran solvents were evaporated under low pressure, and the reaction solution was extracted three times with 20ml of dichloromethane, the combined extracts were dried with anhydrous magnesium sulfate, filtered, and spin-dried. Obtain pure product (chromatographic fluid n-hexane:dichloromethane=4:1R by chromatographic column) f =0.5) as a yellow solid (334 mg, yield 69%).
[0033] 1 H NMR (CDCl 3 -d,δppm):10.12(...
Embodiment 2
[0037] Synthesis of compound 1B
[0038]
[0039] To 4-bromo-7-[4-(diphenylamino)phenyl]-2,1,3-benzo Thiadiazole (458 mg, 1.0 mmol) in a mixed solution of toluene (10 mL) and 2M aqueous sodium carbonate (2 mL). Tetrakis(triphenylphosphine)palladium (1.73mg, 0.0015mmol) was added to the reaction at room temperature, and the temperature was raised to 100°C and refluxed overnight. The reaction solution was cooled to room temperature, most of the toluene and tetrahydrofuran solvents were evaporated under low pressure, and the reaction solution was extracted three times with 20ml of dichloromethane, the combined extracts were dried with anhydrous magnesium sulfate, filtered, and spin-dried. Obtain pure product through chromatographic column (chromatographic liquid n-hexane: dichloromethane=5:1R f =0.6) as a pale yellow solid (363 mg, yield 71%).
[0040] 1 H NMR (CDCl 3 -d,δppm):8.21(d,2H),8.06(d,2H),7.88(d,2H), 7.84(d,1H),7.78(d,1H),7.74-7.70(m,1H), 7.54-7.50(m,1H), 7.34-...
Embodiment 3
[0044] Synthesis of Compound 1C
[0045]
[0046] A solution of (4-methylbenzoate-7-[4-(diphenylamino)phenyl]-2,1,3-benzothiadiazole 513 mg, 1.0 mmol) in THF (10 mL) was added to Saturated methanol solution of sodium hydroxide, stirred overnight at room temperature, steamed tetrahydrofuran and methanol solvent under low pressure, adjusted the pH to 1 with 1 N hydrochloric acid, added 10ml of distilled water, and extracted the reaction solution three times with 10ml of dichloromethane, combined the extracts And dried with anhydrous magnesium sulfate, filtered, and spin-dried. Obtain pure product through column chromatography (chromatographic fluid n-hexane: methylene chloride=1:2R f =0.5) as a yellow solid (454 mg, yield 91%).
[0047] 1 H NMR (CDCl 3 -d,δppm):13.1(s,1H),8.16(d,2H),8.10(d,2H), 8.07-7.93(m,4H),7.41-7.33(m,4H),7.15-7.09(m ,8H). 13 C NMR (CDCl 3 -d, δppm): 167.6, 153.8, 153.7, 148.1, 147.3, 141.5, 133.1, 130.9, 130.7, 130.69, 130.6, 130.2, 130.0, 129.64,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com