Method for administering TRAIL-like protein to continuously inhibit tumor cell growth
A technology for tumor cells and drug delivery methods, which is applied in the field of drug delivery where TRAIL-like proteins continue to inhibit the growth of tumor cells, and can solve problems such as insufficient experimental basis, unexplored intravenous injection, interval, repeated efficacy, and different treatment responses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
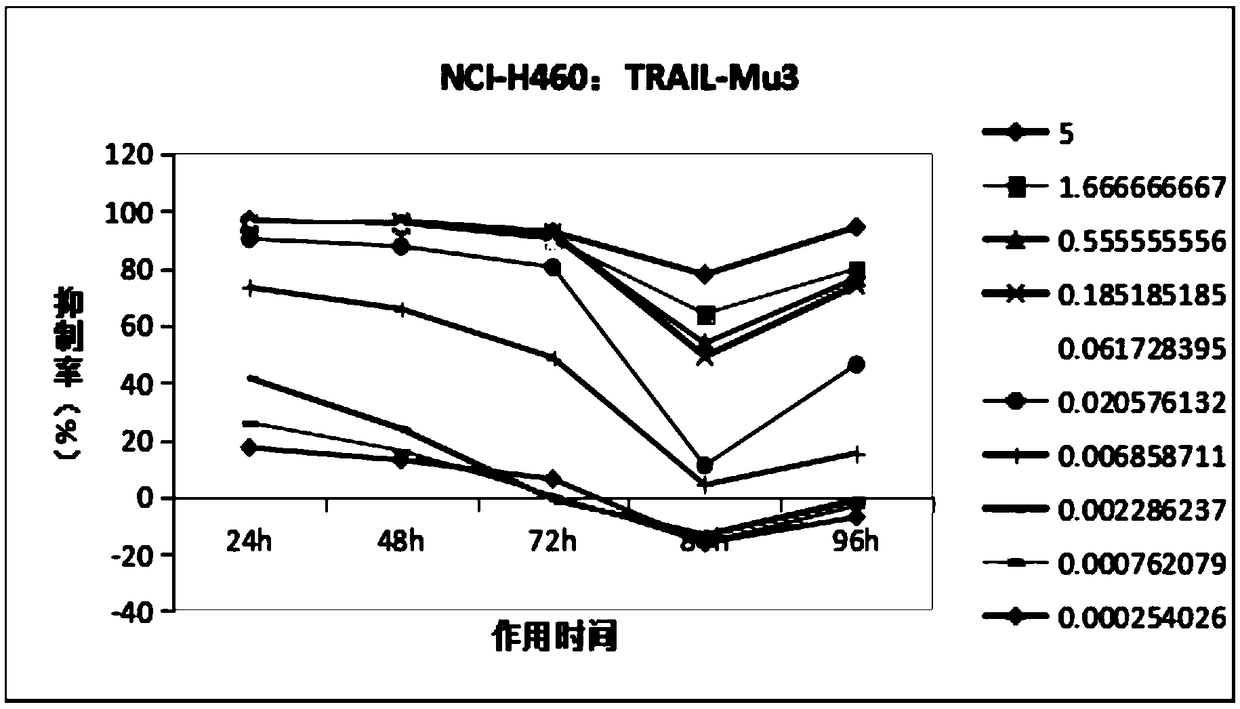
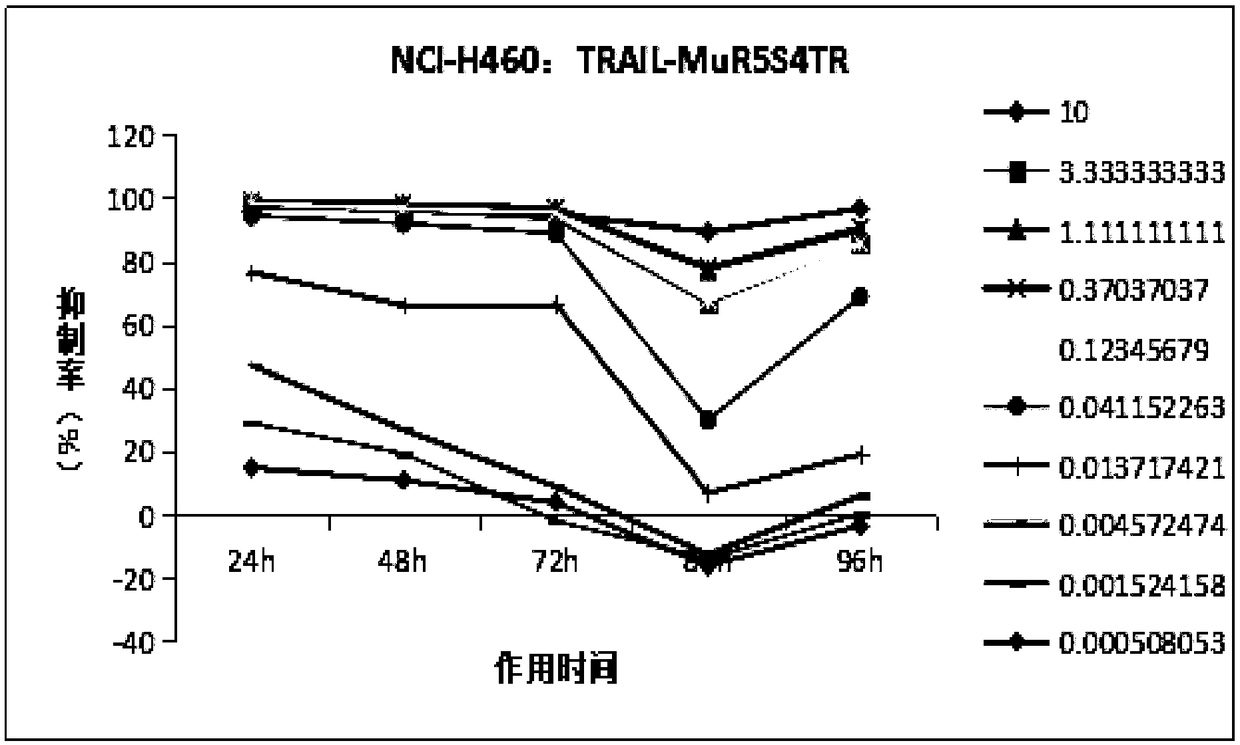
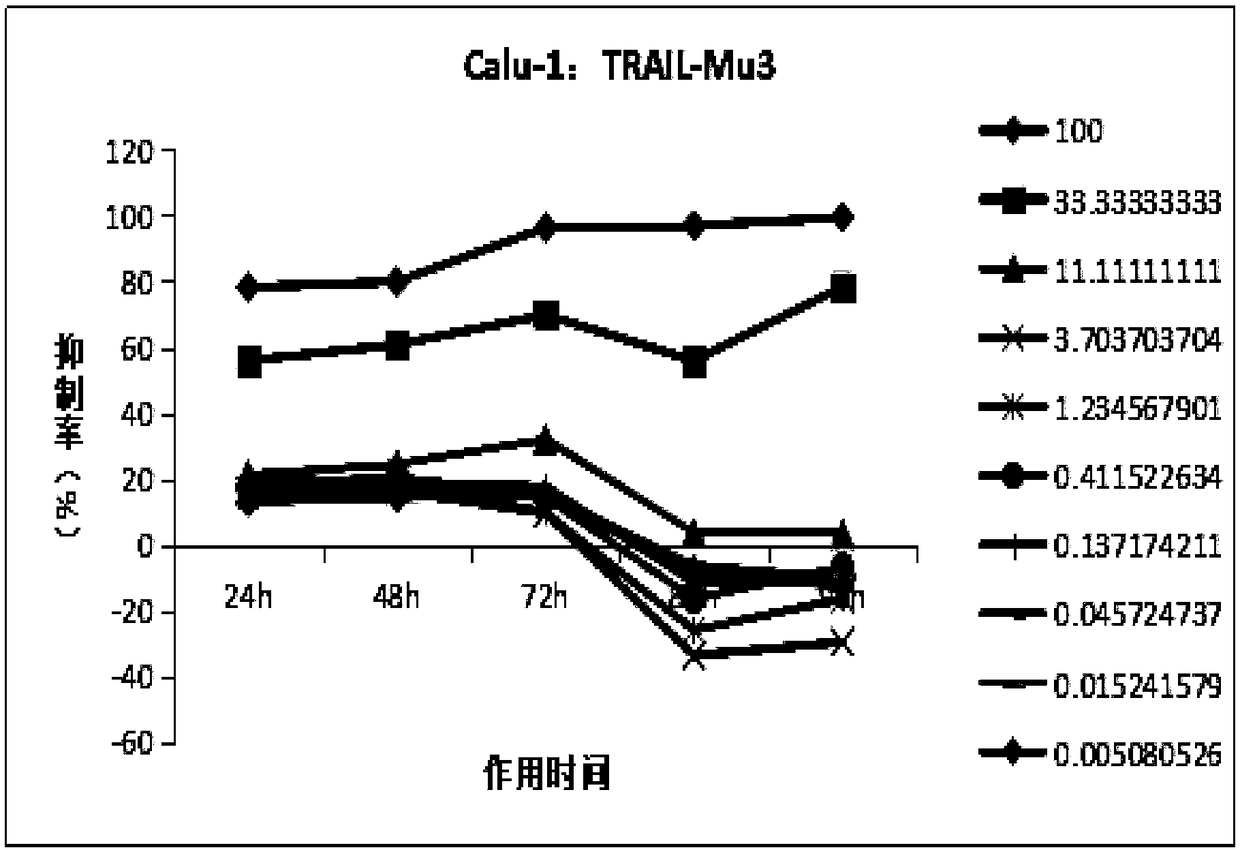
[0095] Study on time-effect relationship of TRAIL-Mu3 and TRAIL-MuR5S4TR inhibiting tumor cell growth in vitro
[0096] 1. Research purpose
[0097] To study the tumor inhibitory effect of different concentrations (doses) of TRAIL-Mu3 and TRAIL-MuR5S4TR on human lung cancer cells NCI-H460, Calu-1, NCI-H1299 in vitro and its relationship with time.
[0098] 2. Experimental materials, reagents and equipment
[0099] 2.1 Preliminary experiment
[0100] 2.1.1 Cell selection: Based on the previous experimental data, (see Table 1) three strains of cells with different degrees of sensitivity were selected for the formal experiment.
[0101] Table 1. In vitro tumor inhibitory effects of TRAIL-Mu3 and TRAIL-MuR5S4TR on seven lung cancer cell lines
[0102]
[0103]
[0104] Based on the data in the above table, considering the effects of TRAIL-Mu3 and TRAIL-MuR5S4TR, a sensitive strain: NCI-H460; an insensitive strain: NCI-H1299; and a moderately sensitive strain: calu-1 were ...
Embodiment 2
[0145] Study on anti-tumor effect of different administration times of TRAIL on human lung cancer NCI-H460 cell xenograft tumor in nude mice
[0146] 1. Purpose of the experiment
[0147] Intravenous injection of 60 mg / kg once a day for 5 consecutive days (Schedule 1: 5 times in total), intravenous injection of 60 mg / kg once a day for 5 consecutive days and then continuous injection for 5 days at intervals of 2 days (Scheme 2: a total of 10 times) or 60mg / kg dose of intravenous injection, once a day, continuous injection for 5 days after an interval of 2 days, and another continuous injection for 5 days after an interval of 2 days again (Scheme 3: a total of 15 times) ) three different administration regimens, and compare the differences in the anti-tumor activity in the human lung cancer NCI-H460 nude mouse xenograft model in vivo at three different administration times.
[0148] 2. Experimental animals
[0149] 2.1 Animal species
[0150] mice.
[0151] 2.2 Varieties
...
Embodiment 3
[0233] Study on the therapeutic effect of TRAIL-Mu3 and TRAIL-MuR5S4TR at different dosing intervals on human lung cancer NCI-H460 xenograft tumor in nude mice
[0234] 1. Purpose of the experiment
[0235] In this study, the human lung cancer NCI-H460 nude mouse xenograft tumor model was used to evaluate the difference in the in vivo anti-tumor activity of TRAIL-Mu3 and TRAIL-MuR5S4TR at different dosing intervals.
[0236] 2. Experimental animals
[0237] 2.1 Animal species
[0238] mice.
[0239] 2.2 Varieties
[0240] Balb / c nude mice.
[0241] 2.3 Gender
[0242] female.
[0243] 2.4 Quantity
[0244] 90 rats were inoculated, and 64 rats were selected.
[0245] 2.5. Age
[0246] 4-6 weeks.
[0247] 2.6. Weight
[0248] 16 ~ 18g ± 20% weight mean.
[0249] 2.7. Animal origin (supplier)
[0250]Shanghai Xipuer-Bikay Laboratory Animal Co., Ltd. (BK), license number SCXK (Shanghai) 2013-0016, animal certificate number: 2008001661519.
[0251] 2.8. Experimental a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com