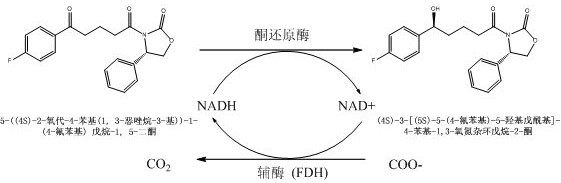
An improved ketoreductase and its application
A reductase and mutant technology, applied in the field of biocatalytic synthesis, can solve the problems of large enzyme consumption, unfavorable scale-up production, and inability to use on a large scale
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]Example 1: Obtaining the recombinant plasmid of the wild-type ketoreductase parent of the T.brockii bacterial strain
[0021] The ketoreductase maternal coding gene of T.brockii strain (GenBank: X64841.1) was obtained through the NCBI Benbank nucleic acid database, and the codons and restriction sites were optimized, and a service provider was entrusted to artificially synthesize the full-length gene and restriction sites at both ends point, and pET28a (+) expression plasmid were digested respectively, gel cutting recovery, connection and transformation Escherichia coli BL21 (DE3) competent cells were spread on the LB agar plate containing 50mg / L kanamycin sulfate, 37 Cultivate overnight. Select several single colonies to LB medium (containing 50mg / L kanamycin sulfate), culture overnight at 37°C, and use a plasmid extraction kit to extract recombinant plasmids, perform PCR and sequencing verification, and obtain the recombination of the positive ketoreductase parent pla...
Embodiment 2
[0022] Example 2: Random mutation of the wild-type ketoreductase parental gene
[0023] According to the content described in Example 1, using the recombinant plasmid containing the wild-type ketoreductase female coding gene of the T.brockii strain as a template, and using Primer 5.0 to design and synthesize two ketoreductase female coding genes according to the T.brockii strain end primers (Table 1), using error-prone PCR technology (see Table 2 for materials and concentrations, and Table 3 for reaction conditions) to obtain linear gene fragments containing a large number of base mutations, these PCR products and pET28a(+) expression plasmids were respectively Enzyme digestion, gel recovery, connection and transformation of Escherichia coli BL21 (DE3) competent cells, spread on LB agar plates containing 50 mg / L kanamycin sulfate, and culture overnight at 37°C.
[0024] Table 1 Random Mutagenesis Primer Sequence
[0025]
[0026] Table 2 50μL error-prone PCR material syste...
Embodiment 3
[0030] Example 3: Cloning and expression of ketoreductase mutants
[0031] In order to facilitate the cloning, expression and identification of ketoreductase mutants, compatible restriction endonuclease sites were designed at the 5' and 3' ends of the gene, and Nde I and Xho I restriction enzymes can be used to separate the target The gene and pET28a(+) (other expression plasmids that can express proteins in E. coli can also be used) are digested and DNA gel is recovered at the same time, and the recovered target gene and the larger fragment of the plasmid are ligated with T4 DNA ligase For the reaction, the ligation product was transformed into Escherichia coli BL21 (DE3) competent cells, and then the transformed competent cells were spread on LB agar plates containing 50 mg / L kanamycin sulfate, and cultured overnight at 37°C.
[0032] Pick a single colony grown on the above-mentioned petri dish and inoculate it in LB liquid medium containing 50mg / L kanamycin sulfate, culture...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com