Recombinant bacillus subtilis and application thereof
A technology of Bacillus subtilis and bacitracin, applied in the field of construction of Bacillus subtilis engineering bacteria, can solve the problems of few reports on biodegradable polypeptide antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: Construction of recombinant shuttle plasmid pWGPapr
[0028] 1. PCR amplification of the polypeptide antibiotic degrading enzyme gene fragment papr.
[0029] Using the genome of Bacillus licheniformis ATCC 14580 as a template, using PaprF and PaprR containing restriction enzyme sites as primers, PCR amplifies the target fragment papr of the polypeptide antibiotic degrading enzyme gene, and the amplified product is detected by 1% agarose gel electrophoresis , the size of the PCR product is 1539bp (the size is 1539bp, including the promoter part, the sequence is shown in SEQ ID NO.1). After purification, restriction endonucleases Xho I and BamH I (Dalian TaKaRa Company) were used for double digestion, purified and recovered for use.
[0030] Upstream primer (PaprF: 5'-CCG CTCGAG ATCTTTCACCCGTTTCTG-3', the underline is the Xho I restriction site);
[0031] Downstream primer (PaprR: 5'-CGC GGATCC TTATGGAGCGGCAGCTTC-3', the underline is the BamH I restric...
Embodiment 2
[0042] Example 2: Construction of recombinant Bacillus subtilis containing recombinant shuttle plasmid pWGPapr.
[0043] Utilize the plasmid extraction kit (Sangon Bioengineering (Shanghai) Co., Ltd.) to extract the recombinant shuttle plasmid pWGPapr from the Escherichia coli DH5α positive transformant containing the recombinant shuttle plasmid pWGPapr constructed in Example 1, and adopt the electroporation method in Under the conditions of voltage 21kV / cm and time constant = 5ms, the recombinant shuttle vector pWGPapr was transformed into Bacillus subtilis WB800 strain, screened on a 50μg / mL kanamycin-resistant LB plate, and obtained after colony PCR and sequencing verification. Bacillus subtilis WB800 / pWBUC01-apr of the recombinant shuttle plasmid pWGPapr, which is Bacillus subtilis WB800dc that degrades polypeptide antibiotics, was deposited in the China Center for Type Culture Collection on November 27, 2017, with a deposit number of It is: CCTCCNO:M 2017732, and the pres...
Embodiment 3
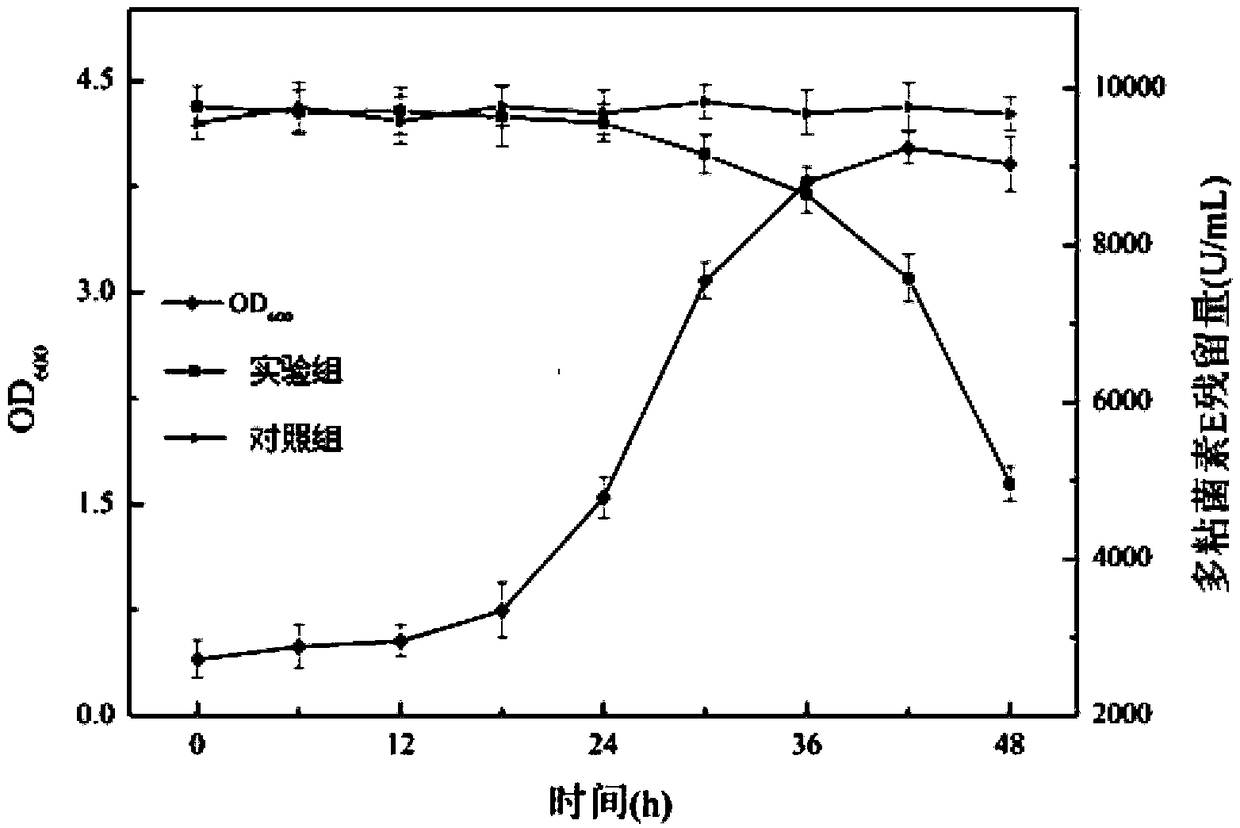
[0045] Example 3: Preliminary study on the degradation ability of Bacillus subtilis WB800dc to polymyxin E, a polypeptide antibiotic.
[0046] 1. Preparation of seed suspension:
[0047] LB liquid medium: Yeast powder 5.0g / L, tryptone 10.0g / L, sodium chloride 10.0g / L, solvent is deionized water, pH 7.0~7.2, filled with 50mL / 250mL triangular flask, sterilized at 121℃ 20min.
[0048] LB solid medium: yeast powder 5.0g / L, tryptone 10.0g / L, sodium chloride 10.0g / L, agar 20.0g / L, solvent is deionized water, pH 7.0~7.2, liquid 100mL / 250mL triangle Bottles, sterilized at 121°C for 20 minutes.
[0049] Streak inoculation of Bacillus subtilis WB800dc cells on LB solid medium containing 50 μg / mL kanamycin for activation, after inverting at 37°C for 24 hours, pick a single colony and inoculate in LB liquid medium, at 37°C, 200r Cultivate for 24 hours / min for further activation; then inoculate into LB liquid medium according to the volume concentration of 8% for expanded culture for 48...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com