A kind of method that carbonyl is reduced to alcohol under room temperature air atmosphere
An air and atmosphere technology, applied in chemical instruments and methods, reduction preparation of oxygen-containing functional groups, preparation of organic compounds, etc., can solve the problems of unfavorable catalyst application, complex catalyst structure, poor substrate applicability, etc., and achieve low cost and toxicity Small, low toxicity effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
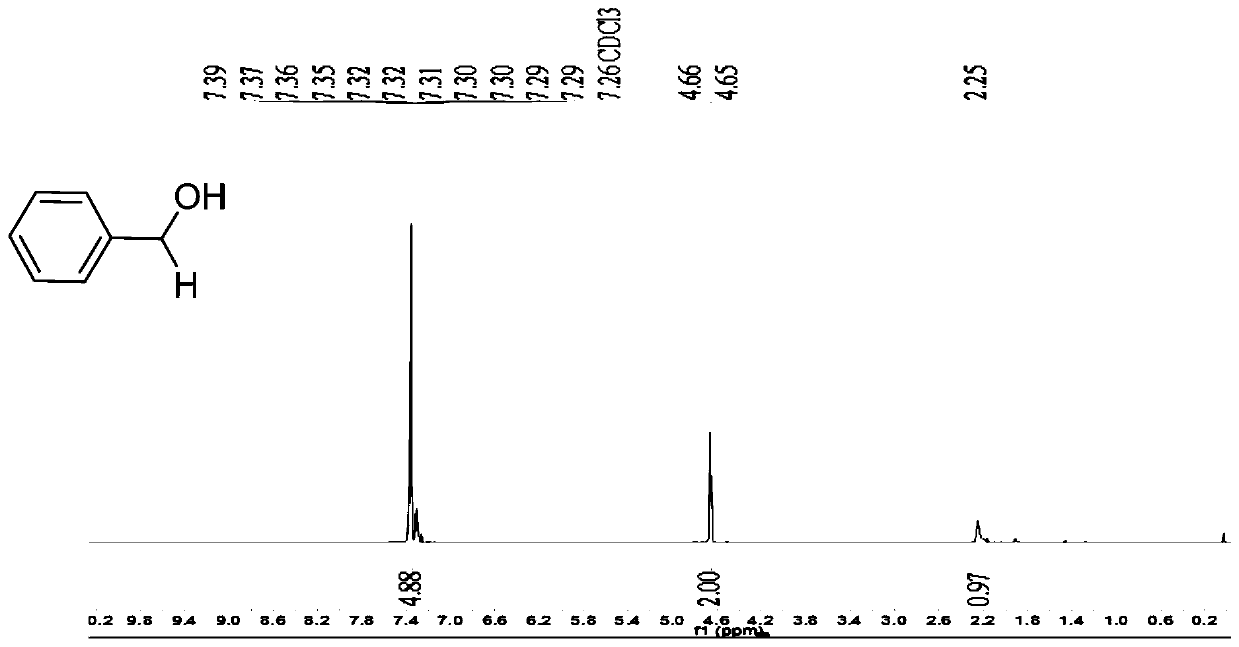
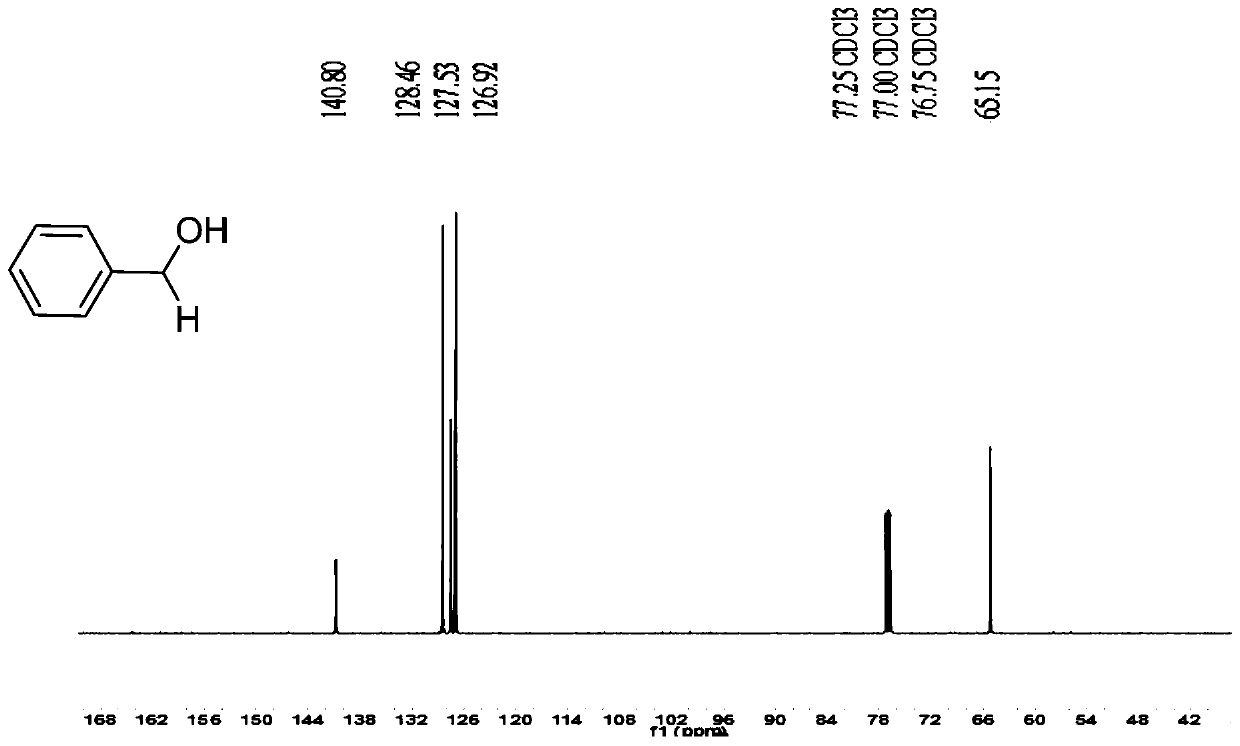
[0043] Embodiment 1: the synthesis of benzyl alcohol
[0044] Take a 25mL reaction bottle and put a magnet into the bottle. Weigh benzaldehyde (0.080g, 0.75mmol), potassium 4-carboxyphenyltrifluoroborate (2.5% eq.4.4mg), add 3mL methanol as solvent to the reaction bottle, and then pipette the triethoxy base silane (2.2eq.306μL), react in air atmosphere at room temperature. The reaction process was detected by TLC. After 8 hours, it was extracted with dichloromethane (3×40mL), the dichloromethane phases were combined, washed with 40mL deionized water, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Color liquid product 64mg, yield 72%; NMR is as follows:
[0045] 1 H NMR (500MHz, CDCl 3 ): δ7.40–7.28(m,5H),4.66(d,J=5.6Hz,2H),2.25(s,1H). 13 C NMR (125MHz, CDCl 3 ): δ140.80, 128.46, 127.53, 126.92, 65.15.
Embodiment 2
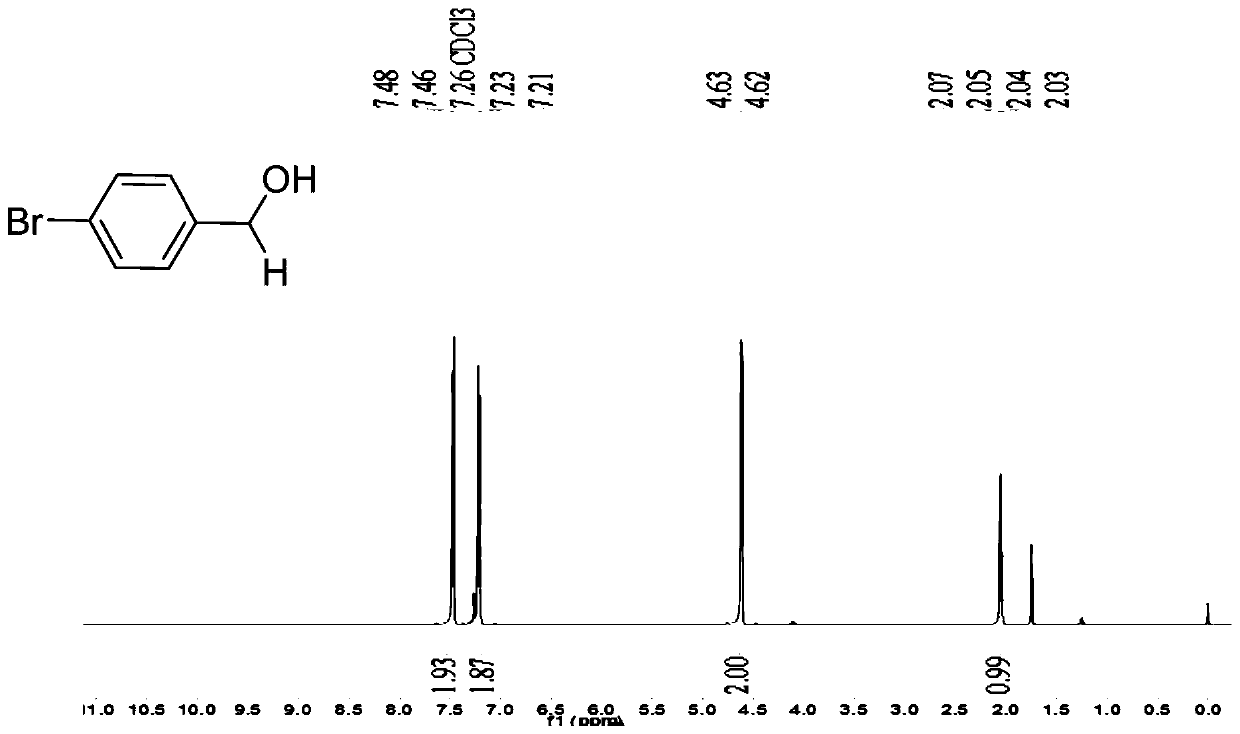
[0046] Embodiment 2: the synthesis of p-bromobenzyl alcohol
[0047] Take a 25mL reaction bottle and put a magnet into the bottle. Weigh p-bromobenzaldehyde (0.139g, 0.75mmol), potassium phenyl trifluoroborate (2.5% eq. 3.5mg), add 3mL methanol as solvent to the reaction bottle, and then pipette the triethoxy Silane (2.2eq.306μL), react in air at room temperature. The reaction process was detected by TLC. After 20 minutes, it was extracted with dichloromethane (3×40mL), the dichloromethane phases were combined, washed with 40mL deionized water, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Column chromatography gave white Solid product 131mg, yield 93%; NMR is as follows:
[0048] 1 H NMR (500MHz, CDCl3 ): δ7.47(d, J=8.3Hz, 2H), 7.22(d, J=8.1Hz, 2H), 4.62(d, J=5.8Hz, 2H), 2.05(t, J=5.9Hz, 1H ). 13 C NMR (125MHz, CDCl 3 ): δ139.70, 131.56, 128.53, 121.38, 64.46.
Embodiment 3
[0049] Embodiment 3: the synthesis of 2,6-dichlorobenzyl alcohol
[0050] Take a 25mL reaction bottle and put a magnet into the bottle. Weigh 2,6-dichlorobenzaldehyde (0.131g, 0.75mmol), potassium phenyltrifluoroborate (2.5%eq.3.5mg), add 3mL methanol as solvent to the reaction bottle, and then pipette Trimethoxysilane (2.2eq.202μL) was reacted in air at room temperature. The reaction process was detected by TLC. After 10 minutes, it was extracted with dichloromethane (3×40mL), the dichloromethane phases were combined, washed with 40mL deionized water, dried over anhydrous sodium sulfate, and the solvent was removed by rotary evaporation. Column chromatography gave white Solid product 120mg, yield 90%; NMR is as follows:
[0051] 1 H NMR (500MHz, CDCl 3 ): δ7.31(d, J=8.1Hz, 2H), 7.20–7.13(m, 1H), 4.94(d, J=5.9Hz, 2H), 2.33(t, J=6.3Hz, 1H). 13 CNMR (125MHz, CDCl 3 ): δ135.89, 135.58, 129.72, 128.38, 60.05.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com