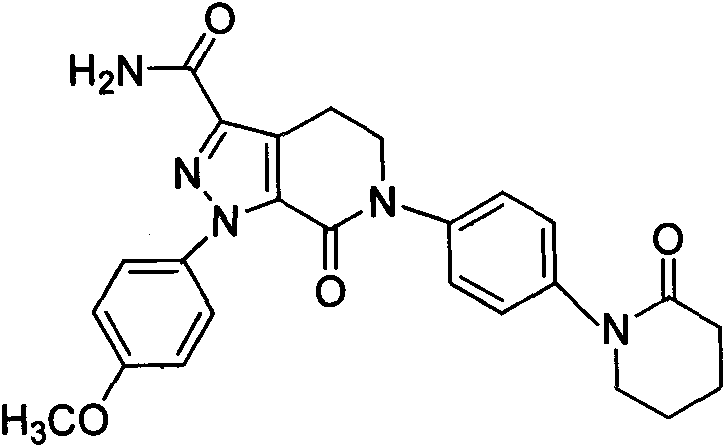
A kind of apixaban nano-suspension and preparation method thereof
A nanosuspension, apixaban technology, applied in the field of medicine, can solve problems such as low bioavailability, achieve the effects of less dosage, improved adhesion, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
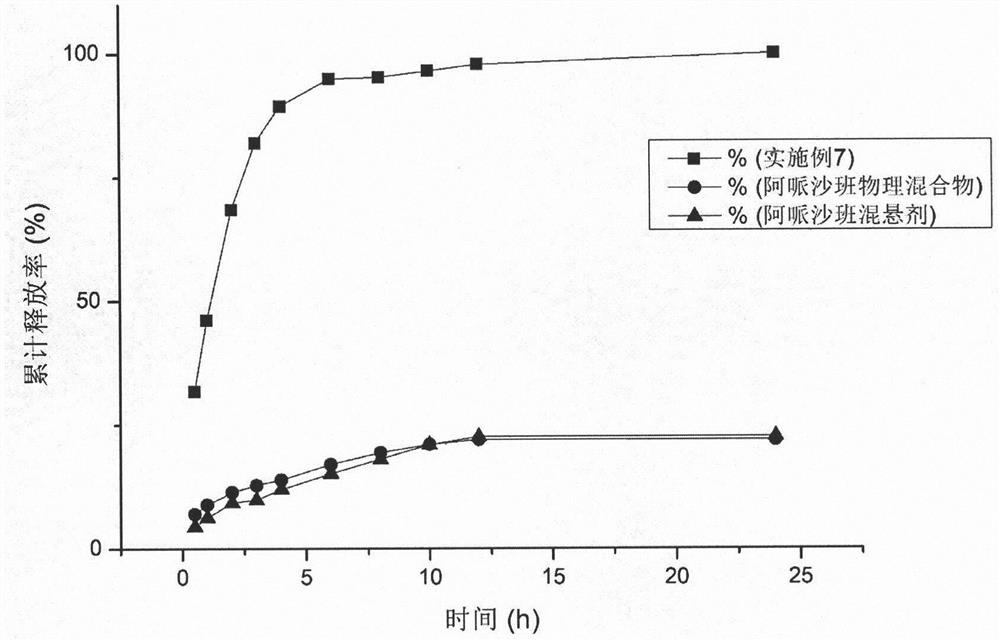
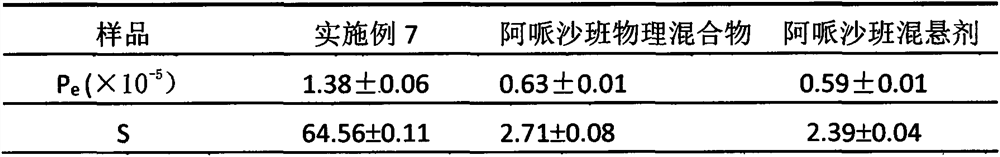
[0027] Weigh 20 mg of apixaban in 35 ml of water, ultrasonically treat for 10 minutes to fully disperse the drug in water; use a high-speed disperser to cut at 10,000 rpm for 5 minutes to obtain a coarse suspension; perform high-pressure homogenization on the above coarse suspension , first homogenized twice at 800 bar, and then homogenized 12 times at 1800 bar pressure to obtain the apixaban nanosuspension. The measured particle size of the nanosuspension was 754nm, and the polydispersity index (PDI) was 0.378.
Embodiment 2
[0029] Weigh 5 mg of PVPK30 in 50 ml of water, add 20 mg of apixaban under stirring, and ultrasonically treat for 10 min to fully disperse the drug in water; use a high-speed disperser to shear at 10,000 rpm for 5 min to obtain a coarse suspension; The suspension was subjected to high-pressure homogenization, first at 800 bar for 2 times, and then at 1800 bar for 12 times to obtain the apixaban nanosuspension. The measured particle size of the nanosuspension was 386.7nm, and the polydispersity index (PDI) was 0.167.
Embodiment 3
[0031] Weigh 1 mg of sodium taurocholate into 35 ml of water, add 20 mg of apixaban under stirring, and ultrasonically treat for 10 min to fully disperse the drug in water; use a high-speed disperser to shear at 10,000 rpm for 5 min to obtain a coarse suspension; The above coarse suspension was subjected to high-pressure homogenization, first at 800 bar for 2 times, and then at 1800 bar for 12 times to obtain the apixaban nano-suspension. The measured particle size of the nanosuspension was 401.6 nm, and the polydispersity index (PDI) was 0.11.
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com