Primers and probe for detecting porcine epidemic diarrhea virus based on digital PCR technology, kit and a method thereof
A technology for porcine epidemic diarrhea and technology detection, applied in the direction of microorganism-based methods, biochemical equipment and methods, recombinant DNA technology, etc., to achieve broad application prospects and industrialization prospects, high sensitivity, and avoid economic losses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Establishment of a kit for the absolute quantitative detection of porcine epidemic diarrhea virus based on digital PCR technology
[0045]A kit for the absolute quantitative detection of porcine epidemic diarrhea virus based on digital PCR technology, including a primer set, 2×RT-ddPCR Supermix, RNase-free distilled water, virus total RNA extraction reagent, positive control and negative control.
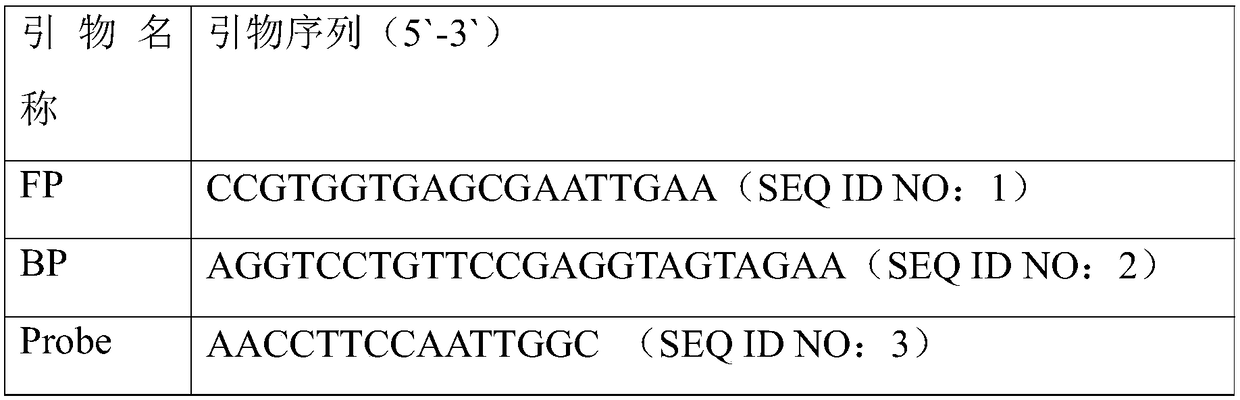
[0046] (1) Design of primers for digital PCR amplification: primers were designed with the specific conserved sequence of porcine epidemic diarrhea virus as the target gene. The primer sequences are listed in Table 1.
[0047] Table 1 Primer sequence list
[0048]
[0049] (2) The molar ratio of FP primer, BP primer and probe in the primer set is 3:3:2.
[0050] (3) 2×RT-ddPCR Supermix contains: 2×One-step RT-ddPCR Supermix and 25mMManganous acetate.
[0051] (4) The positive control is porcine epidemic diarrhea virus cell culture, and the negative control is ...
Embodiment 2
[0053] Example 2 The detection method of absolute quantitative detection of porcine epidemic diarrhea virus based on digital PCR technology
[0054] The method utilizing the kit of embodiment 1 to detect porcine epidemic diarrhea virus may further comprise the steps:
[0055] (1) Extraction of viral RNA:
[0056] 1) Take the sample to be tested and the positive control, add 600ul lysate A respectively, vortex and mix for 20 seconds, and let stand at room temperature for 10 minutes;
[0057] 2) Transfer the mixture to an adsorption column and centrifuge at 12,000×g for 30-60 seconds;
[0058] 3) Discard the liquid in the collection tube, add 500ul washing solution B to the adsorption column, and centrifuge at 12,000×g for 30-60 seconds;
[0059] 4) Discard the liquid in the collection tube, add 500ul washing solution C to the adsorption column, and centrifuge at 12,000×g for 30-60 seconds;
[0060] 5) Discard the liquid in the collection tube, and centrifuge at 12,000×g for ...
Embodiment 3
[0079] Example 3 specificity verification
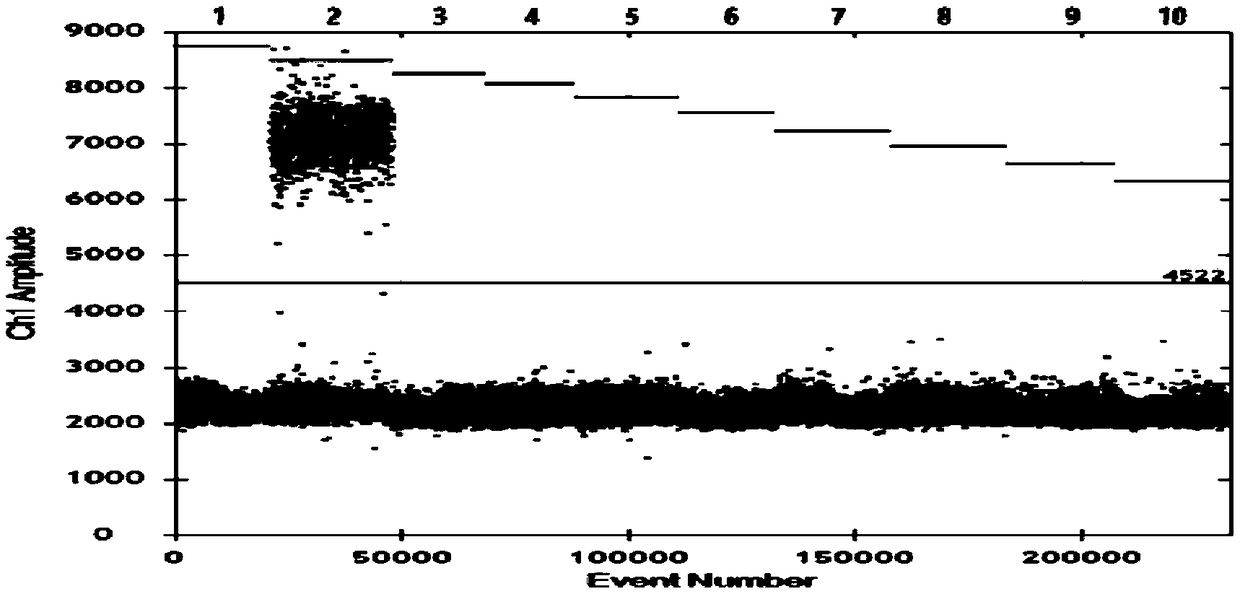
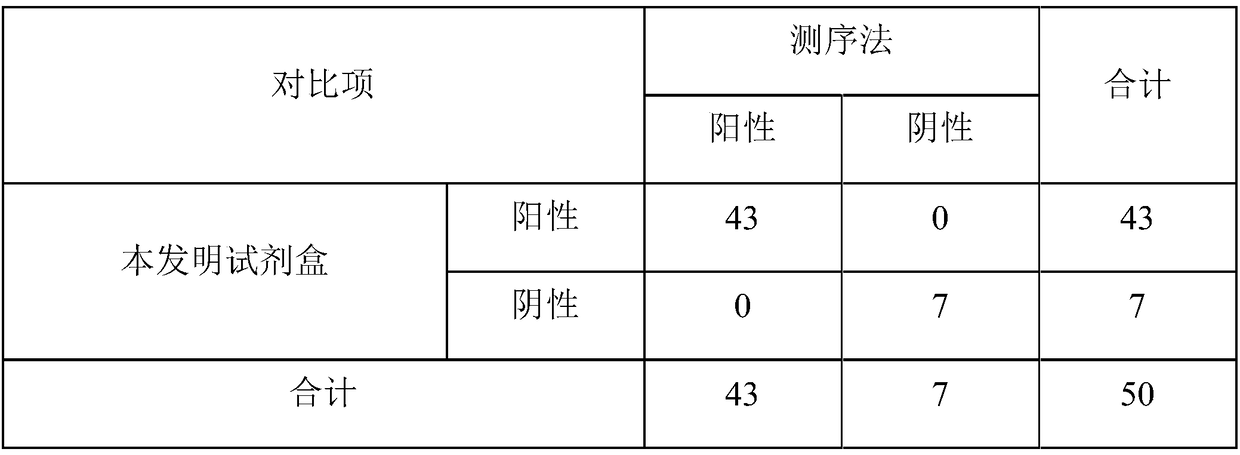
[0080] Use the kit of the present invention to detect serum samples of healthy pigs, clinically obtained pseudorabies virus, porcine respiratory and reproductive syndrome American type virus, swine fever virus, porcine circovirus, Haemophilus parasuis, Streptococcus suis, porcine pleura There are a total of 8 samples of Actinobacillus pneumoniae samples, all of which have been verified by sequencing methods, and the test results can be found in figure 1 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com