A kind of osteopontin characteristic peptide and its application
A technology of osteopontin and characteristic peptides, which is applied in the direction of specific peptides, animal/human proteins, instruments, etc., to achieve the effect of ensuring accuracy and good linearity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
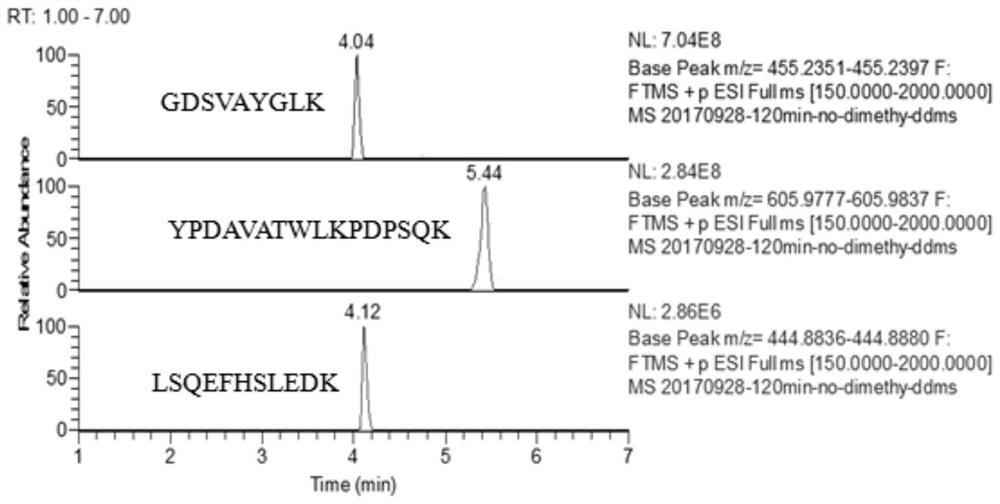
[0048] 1. Search and determination of characteristic peptides
[0049] The selection of specific peptides is a key step in targeted proteomics detection technology, which is directly related to the accuracy of quantitative methods. In the process of selecting specific peptides, the theoretical database is usually combined with the actual sample detection data for analysis, and bioinformatics software is used to assist in the prediction and calculation of protein hydrolysis products.
[0050] The present invention selects Uniprot database and Peptide Mass tool to osteopontin OPN (as in cow, yak, buffalo, sheep, goat milk) figure 1 shown) for prediction and calculation of possible trypsin-digested peptides.
[0051] In order to verify whether there are theoretically determined peptides in the actual samples, the present invention pre-treats five milk products (i.e. samples), respectively, and directly adds formic acid to terminate the reaction after enzymatic hydrolysis, and th...
Embodiment 2
[0106] 1. Standard curve, recovery rate and precision for internal standard peptide 1
[0107] (1) Establishment of standard curve
[0108] Accurately pipette 10 μL, 25 μL, 50 μL, 75 μL, 100 μL of osteopontin-labeled peptide solution (1 μg / mL), add 50 μL of internal standard peptide 1 solution (1 μg / mL), and then add 940 μL, 925 μL, 900 μL of 0.1% formic acid aqueous solution , 875 μL, and 850 μL by vortex mixing.
[0109] Gained solution detects and analyzes by the liquid chromatography described in the 5th part of embodiment 1 and the mass spectrometry condition, each sample is detected in parallel 6 times, and the gained result makes standard curve as follows Figure 5 As shown, the standard curve formula: y=0.024985x-0.041725, R 2 =0.999, where x is the concentration (ng / mL) of the osteopontin tag peptide, and y is the ratio of the peak area of the sample to the peak area of the internal standard peptide 1.
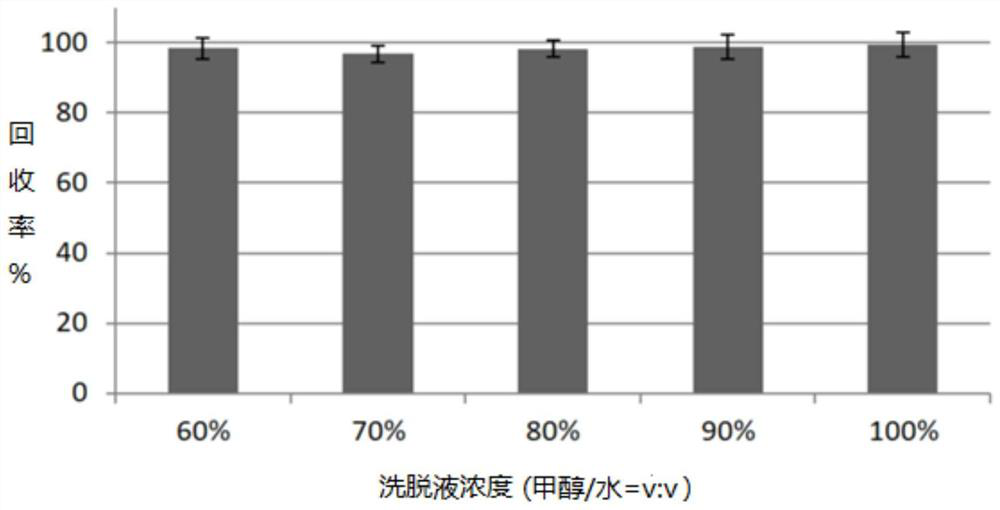
[0110] (2) Recovery rate and precision
[0111] The accu...
Embodiment 3
[0123] Example 3 Detection of Osteopontin Content in Milk and Dairy Products Using Internal Standard Peptide 1
[0124] Cow's milk, buffalo's milk, yak's milk, goat's milk, sheep's milk and formula milk powder were selected as the research objects to determine the content of osteopontin in milk and dairy products. The specific method is as follows:
[0125] (1) Liquid milk: Take 50 μL of liquid milk in a 2 mL plastic tube, add 950 μL of ultrapure water, and vortex to mix.
[0126] Milk powder sample: Weigh 2.5g of milk powder sample to dissolve, transfer to a volumetric flask and dilute to 50mL. Take 100 μL of liquid milk in a 2 mL plastic tube, add 900 μL of ultrapure water, and vortex to mix.
[0127] (2) Accurately draw 300 μL of sample solution, add 4.2 mL of sodium bicarbonate (100 mmol / L) solution, vortex and mix well, add 60 μL of DTT solution (500 mmol / L), react at a constant temperature of 70 °C for 30 minutes, take it out and cool to room temperature, add 180 μL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com