Low-dosage atypical antipsychotic drug composition
An antipsychotic drug and atypical technology, applied in the field of medicine, can solve the problems of patients with residual negative symptoms, reduce patient compliance, shorten life expectancy, etc., achieve the effect of reducing medical costs, reducing cardiac side effects, and eliminating negative symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] According to the conventional capsule preparation method, 62.5g of ziprasidone, 125g of sertraline hydrochloride, 300g of cornstarch, 300g of hydroxypropyl cellulose, 120g of magnesium oxide, 60g of talcum powder, and 32.5g of micropowder silica gel were mixed to form granules, and The filling amount of each 200 mg is packed into capsules to make 5000 capsule preparations, each capsule preparation contains 12.5 mg of ziprasidone and 25 mg of sertraline hydrochloride.
Embodiment 2
[0050] Adopt conventional preparation method, ziprasidone 40g, sertraline hydrochloride 40g, cornstarch 80g, carboxymethyl starch sodium 100g, talcum powder 20g, magnesium stearate 10g, microcrystalline cellulose 10g are mixed to make powder, use The powder was compressed by a rotary tablet press to make 2000 tablets, each 150 mg, containing 20 mg of ziprasidone and 20 mg of sertraline hydrochloride.
Embodiment 3
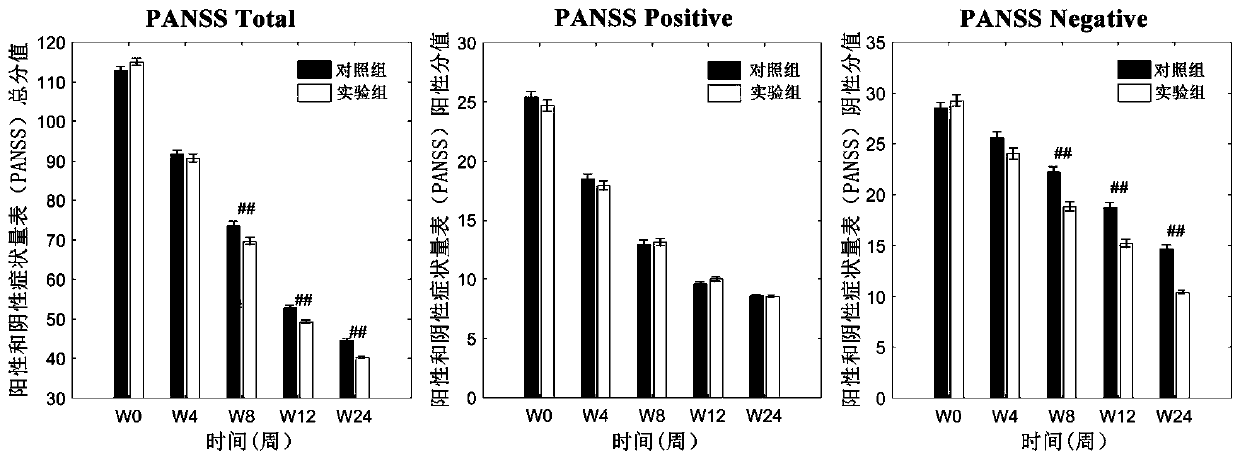
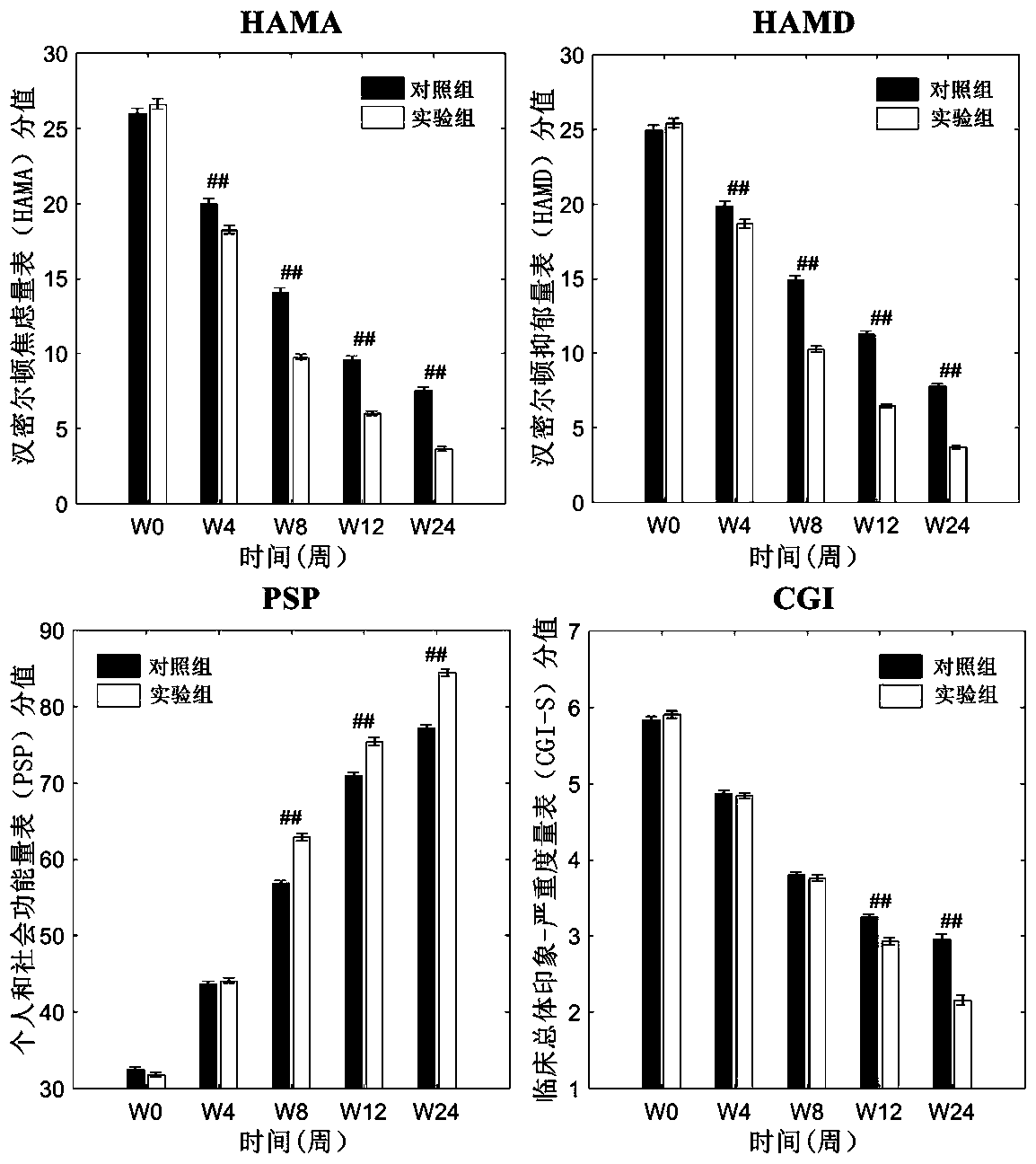
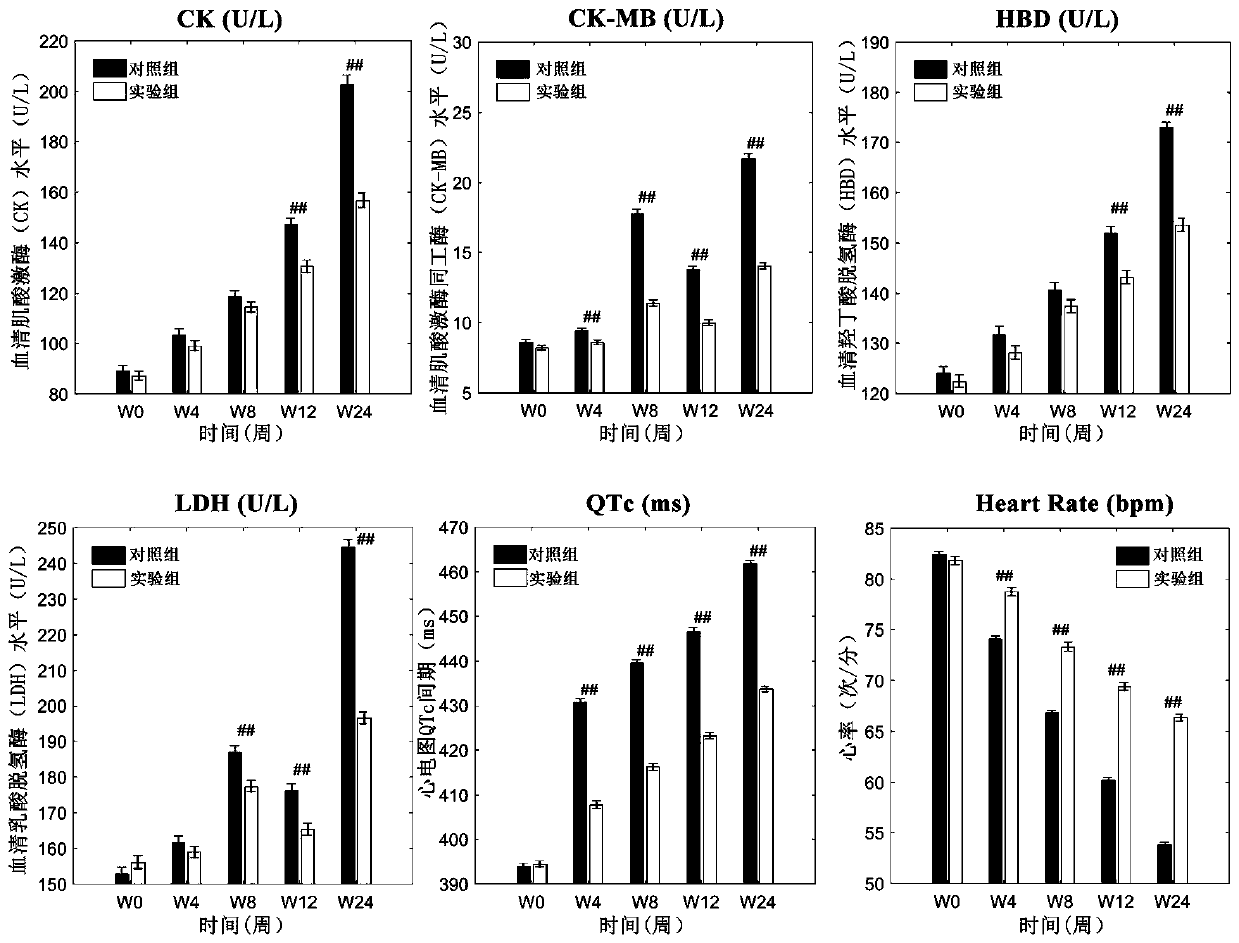
[0052] Screen and collect 468 cases of schizophrenia patients meeting the inclusion criteria and exclusion criteria to evaluate the effect of the clinical trial of the pharmaceutical composition of the present invention, and divide the subjects into an experimental group and a control group at random, with 234 cases in each group.
[0053] Inclusion criteria: 1) Meet the diagnostic criteria for schizophrenia in DSM-IV; 2) Educational years ≥ 9 years, right-handed, Han nationality, 3) Age 18-60 years old; 4) Have not received any medical treatment 2 weeks before enrollment 5) half a year ≤ total disease duration ≤ 30 years; 6) PANSS score ≥ 60 points; 7) signed the informed consent.
[0054]Exclusion criteria: 1) Those who are known to be allergic to the drugs used, those who have recent pregnancy plans or breastfeeding; 2) Recent acute myocardial infarction, decompensated heart failure, and a history of prolonged QT interval (including congenital long QT interval 3) Those who ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com