High performance liquid chromatography method for detecting characteristic chromatograms of herbal materials, decoction pieces, standard decoction and formula granules of dried leaves of Nelumbo nucifera Gaertn.
A detection method and a technology of formula particles, applied in the field of HPLC detection, can solve the problems of different detection methods, difficult overall evaluation and control of the process, difficult delivery, etc., and achieve stable overall quality, high accuracy and good repeatability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
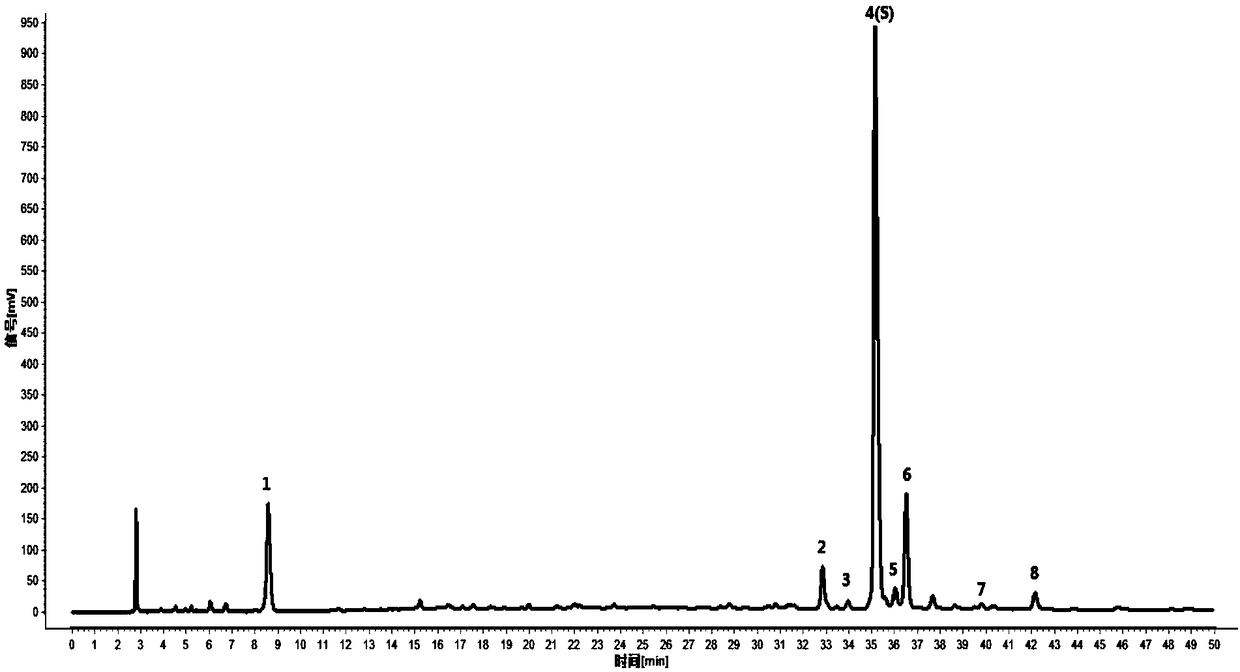
[0043] Embodiment 1 The detection method of the present invention is used for the detection of the HPLC characteristic spectrum of the lotus leaf medicinal material
[0044]1 Preparation of reference substance solution: take appropriate amount of reference substances of nuciferin, rutin, hyperin, and vetrin, and add methanol to prepare reference solutions containing 16, 200, 100, and 30 μg per 1 ml, respectively.
[0045] 2 Preparation of the reference medicinal material solution: take 1g of the lotus leaf reference medicinal material, accurately weigh it, put it in a stoppered Erlenmeyer flask, accurately add 50ml of water, weigh it, decoct it for 45 minutes, let it cool, weigh it again, and make up the reduced amount with water. Lost weight, shake well, filter, that is.
[0046] 3 Preparation of the test solution: take 1g of the lotus leaf medicinal material to be tested, accurately weigh it, place it in a stoppered conical flask, add 50ml of water accurately, weigh it, deco...
Embodiment 2
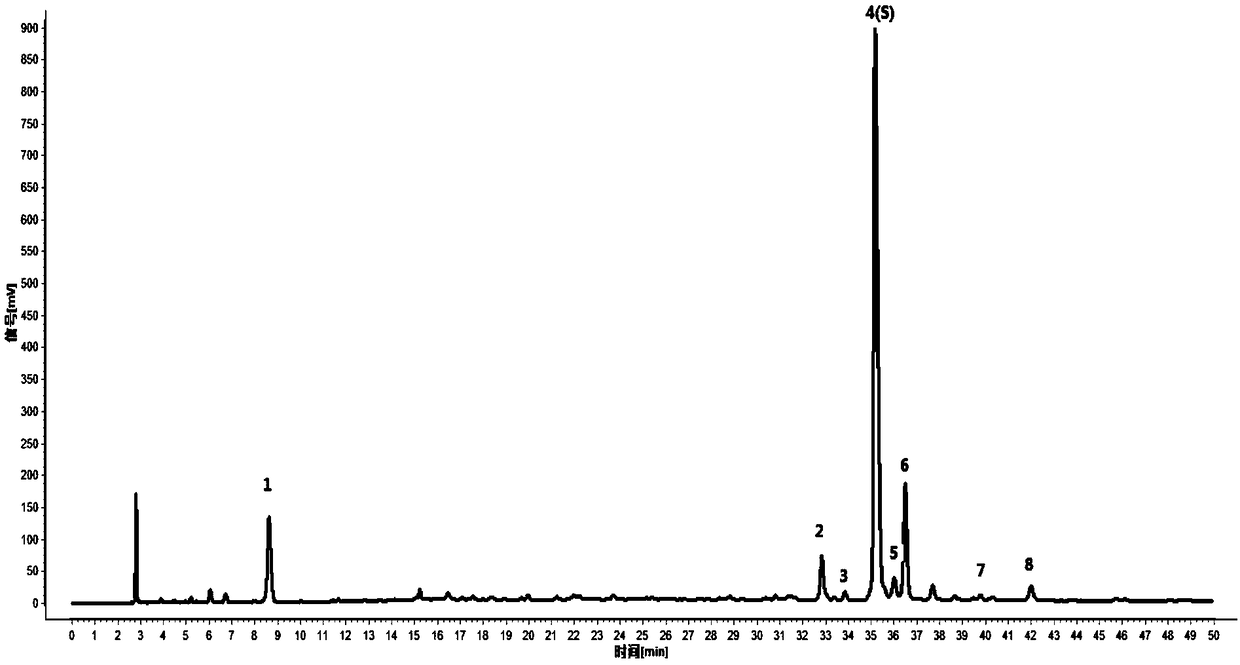
[0051] Example 2 The detection method of the present invention is used for the detection of the HPLC characteristic spectrum of lotus leaf decoction pieces
[0052] 1 Preparation of reference substance solution: take appropriate amount of reference substances of nuciferin, rutin, hyperin, and vetrin, and add methanol to prepare reference solutions containing 16, 200, 100, and 30 μg per 1 ml, respectively.
[0053] 2 Preparation of the reference medicinal material solution: take 1g of the lotus leaf reference medicinal material, accurately weigh it, put it in a stoppered conical flask, accurately add 25ml of water, weigh it, decoct for 30 minutes, let it cool, weigh it again, and make up the reduced amount with water. Lost weight, shake well, filter, that is.
[0054] 3 Preparation of the test solution: take 1g of lotus leaf decoction pieces to be tested, accurately weigh, put in a stoppered Erlenmeyer bottle, add 25ml of water accurately, weigh, decoct for 30 minutes, let cool...
Embodiment 3
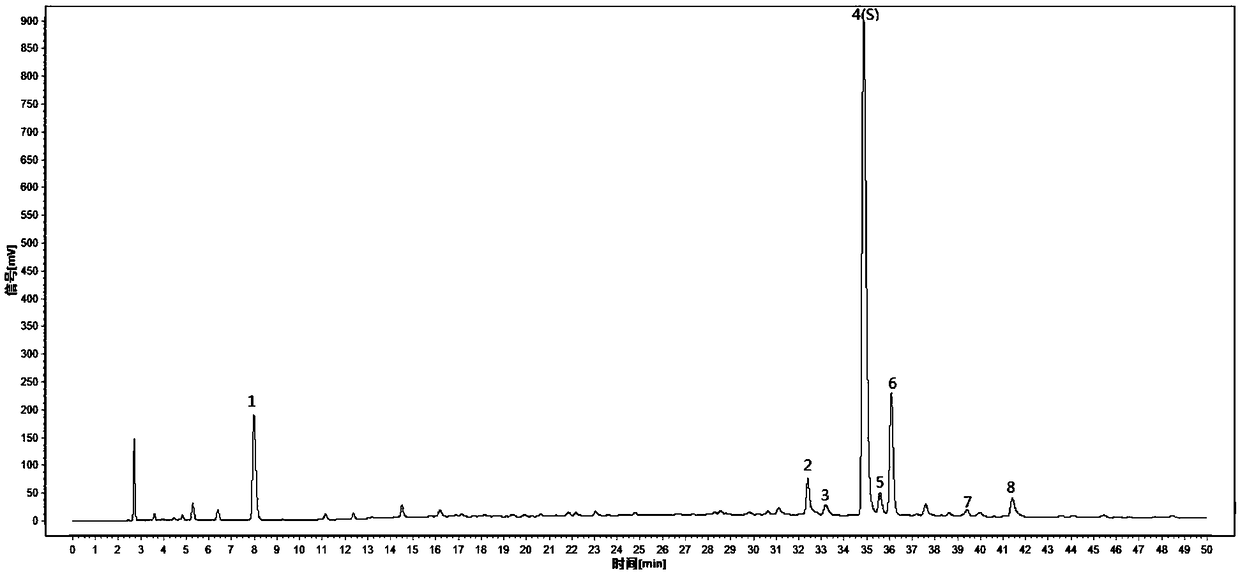
[0057] Embodiment 3 The detection method of the present invention is used for the detection of lotus leaf standard decoction HPLC characteristic spectrum
[0058] 1 Preparation of reference substance solution: take appropriate amount of reference substances of nuciferin, rutin, hyperin, and vetrin, and add methanol to prepare reference solutions containing 16, 200, 100, and 30 μg per 1 ml, respectively.
[0059] 2 Preparation of the reference medicinal material solution: take 1g of the lotus leaf reference medicinal material, accurately weigh it, put it in a stoppered conical flask, accurately add 25ml of water, weigh it, decoct for 30 minutes, let it cool, weigh it again, and make up the reduced amount with water. Lost weight, shake well, filter, that is.
[0060] 3 Preparation of the test solution: take 0.2g lotus leaf standard decoction to be tested, accurately weigh it, put it in a stoppered Erlenmeyer flask, accurately add 10ml of water, weigh it, ultrasonically treat it ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com