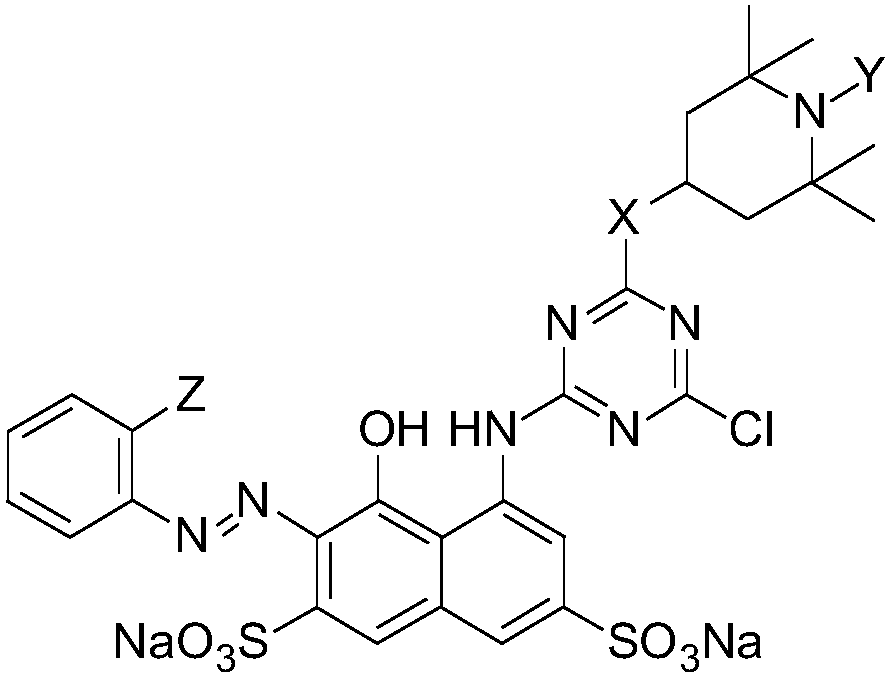
Red sunproof K-type active dye and preparation method thereof
A technology of reactive dyes and light fastness, which is applied in the direction of reactive dyes, azo dyes, organic dyes, etc. It can solve the problems of lack of dye compounding and use, achieve excellent color fastness, improve light resistance, and simple synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1, a kind of red sunfast reactive dye and preparation method thereof, carries out following steps successively:
[0035] 1) Dissolve 0.01mol X-type reactive dye C.I. reactive red 1 in 40mL water to obtain an aqueous reactive dye solution;
[0036] Add 0.01mol of 1,2,2,6,6-pentamethylpiperidinol dissolved in 20mL of acetone to the above reactive dye aqueous solution, then add 0.01mol of potassium carbonate as an acid-binding agent, at 40°C Reaction for 4 hours;
[0037] 2), after completion of the reaction, add 5% (mass %) HCl solution in the reaction solution to adjust the pH to 1, so that the red product is separated out; after filtering, the filter cake is pickled (the mass concentration is 5% hydrochloric acid aqueous solution Pickling, each consumption is 50ml, pickling 3 times altogether), drying (50 DEG C is dried to constant weight), obtains red K-type reactive dye.
[0038] The structural formula of this red K-type reactive dye is as follows:
[00...
Embodiment 2
[0041] Embodiment 2, a kind of red sunfast reactive dye and preparation method thereof, carries out following steps successively:
[0042] 1) Dissolve 0.01mol X-type reactive dye C.I. reactive red 2 in 40mL water to obtain an aqueous reactive dye solution;
[0043] Add 0.01mol of 2,2,6,6-tetramethylpiperidinamine dissolved in 10mL of acetone to the above reactive dye aqueous solution, then add 0.01mol of potassium carbonate as an acid-binding agent, and react at 50°C for 2 Hour;
[0044] 2), after completion of the reaction, add 5% (mass %) HCl solution in the reaction solution to adjust the pH to 1, so that the red product is separated out; Washing, each consumption is 50ml, altogether pickling 3 times) and drying (50 ℃ drying to constant weight), obtains red K-type reactive dye.
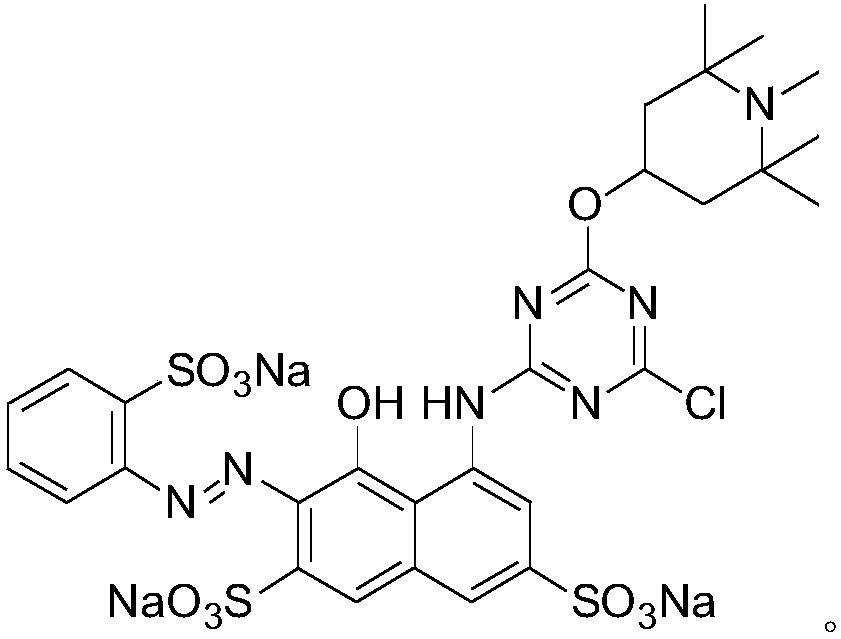
[0045] The structural formula of this red K-type reactive dye is as follows:
[0046]
[0047] 1 H NMR (400MHz, DMSO-d6): δ8.12(s,1H),7.93(d,2H),7.84(s,1H),7.46(m,3H),7.28(s,1H),4.03(m ,2H)...
Embodiment 3
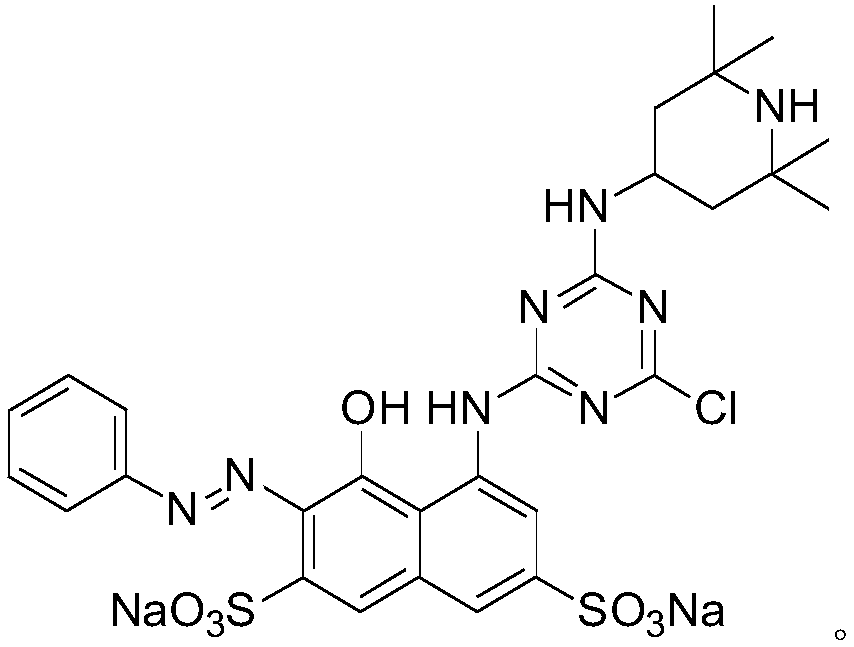
[0048] Embodiment 3, change the 1,2,2,6,6-pentamethylpiperidinol in the embodiment 1 into 2,2,6,6-tetramethylpiperidinamine, the molar dosage remains unchanged; the rest are the same In Example 1.
[0049] The structural formula of the resulting product is:
[0050] 1H NMR (400MHz, DMSO-d6): δ8.21(d,1H),8.13(m,2H),7.84(s,1H),7.72(t,1H),7.49(t,1H),7.26(s ,1H), 4.01(m,2H), 2.63(m,1H), 2.02(s,1H), 1.61(d,4H), 1.15(s,12H); ESI MS(m / z,%): 813.1 ([M-Na] - ,100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com