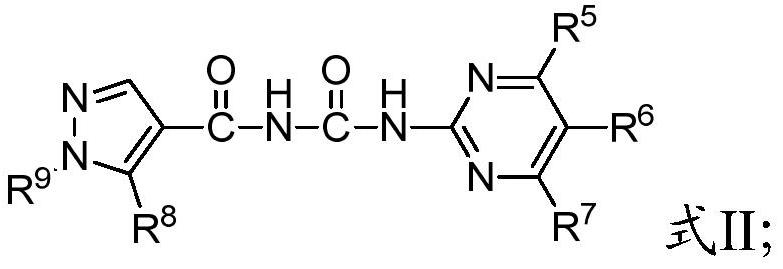
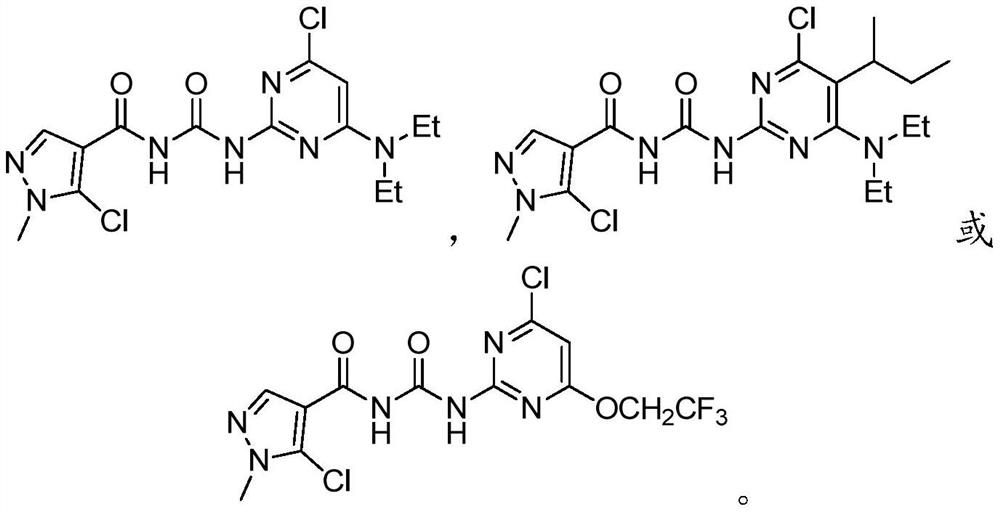
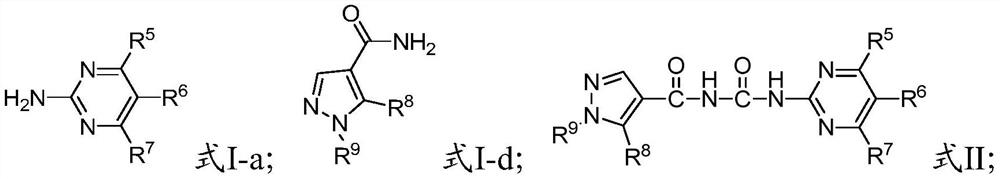A kind of benzoyl pyrimidine urea compound and its preparation and application
A compound, pyrimidine urea technology, applied in the field of new benzoyl pyrimidine urea compounds and their preparation and application, can solve the problems of death, domestic fish hazards, etc., and achieve excellent armyworm killing and broad-spectrum antifungal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] (1) Preparation of 4,6-bis(2,2,2-trifluoroethoxy)pyrimidin-2-amine (I-a)
[0061] Under the protection of inert gas, add 12mL of dry THF to a 100mL three-neck flask containing sodium hydride (0.35g, 14.6mmol), and slowly add 2,2,2-trifluoroethanol dropwise when the temperature of the reaction system drops to 0°C (1.5 g, 15.2 mmol). When the reaction system stopped emitting gas and the reaction solution became clear, 2-amino-4,6-dichloropyrimidine (1.0 g, 6.1 mmol) was added into the three-necked flask. The temperature of the reaction system was raised to 62° C. for 15 h, and the reaction was monitored by TLC until the reaction was complete. Stop heating, cool the reaction system to room temperature, add 1mol L -1 hydrochloric acid solution to quench the reaction. Extract with ethyl acetate, separate the organic phase, wash the organic phase twice with sodium bicarbonate solution, and dry over anhydrous magnesium sulfate. After filtration, the filtrate was spin-dried...
Embodiment 2
[0097] (1) Preparation of 4-chloro-6-morpholinopyrimidin-2-amine (I-g)
[0098] Add cuprous iodide (5.8mg, 0.031mmol), potassium carbonate (0.41g, 2.9mmol), morpholine (0.21g, 2.4mmol) and 2-amino-4, 6-Dichloropyrimidine (0.40g, 2.4mmol) was first stirred at room temperature for 30min, then the reaction system was heated to 110°C, and the reaction was monitored by TLC. After the reaction was completed, the reaction system was cooled to room temperature, washed with water, extracted with ethyl acetate, and the organic phase was dried with anhydrous magnesium sulfate. The solvent was spun off under reduced pressure and passed through a silica gel column to obtain a white solid with a yield of 79.1%.
[0099] mp 210-212°C. 1 H NMR(500MHz,Chloroform-d)δ5.94(s,1H,ArH),5.00(s,2H,NH 2 ),3.79–3.68(m,4H,morpholine),3.55(t,J=4.9Hz,4H,morpholine).
[0100] The intermediates shown in Table 2 below were prepared according to the above method.
[0101] Table 2 Structure and Characteriz...
Embodiment 3
[0120] (1) Preparation of N-((4,6-bis(2,2,2-trifluoroethoxy)pyrimidin-2-yl)thiocarbamoyl)-2-chlorobenzamide (31)
[0121] 2-Chlorobenzoyl isothiocyanate (988 mg, 5 mmol), compound I-a (1.4 g, 5 mmol) and NBS (10 mol%) were mixed and dissolved in 5 mL of anhydrous MeCN. The reaction was then heated to reflux for 4 hours. When the reaction was complete, it was cooled to room temperature, diluted with 20 mL of EtOAc, washed twice with brine (20 mL), washed with anhydrous Na 2 SO 4 Dry and evaporate in vacuo. Finally, the residue was purified by silica gel column chromatography to afford compound 31 as a yellow solid in 53% yield, mp 93-95°C. 1 H NMR (500MHz, DMSO-d 6 )δ12.84(s,1H,NH),12.45(s,1H,NH),7.71–7.62(m,1H,Ph),7.56(dd,J=6.5,1.7Hz,2H,Ph),7.51– 7.42(m,1H,Ph),6.51(s,1H,ArH),5.11(q,J=8.9Hz,4H,OC H 2 CF 3 ). 13 CNMR (126MHz, DMSO) δ178.26, 170.77, 168.24, 156.18, 135.15, 133.26, 131.05, 130.65, 130.34, 128.15, 125.61, 88.16, 63.87, 63.59, 63.32, 63.04. 16 h 11 CIF 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com