Schizophyllan modified sodium hyaluronate microsphere gel, preparation method of sodium hyaluronate microsphere gel, and application of sodium hyaluronate microsphere gel in cosmetics
A technology of sodium hyaluronate and schizophyllan, applied in the direction of cosmetics, cosmetic preparations, medical preparations containing active ingredients, etc., can solve the problems of retention, difficulty in improving skin absorption rate and absorption effect, and achieve small size, Excellent moisture retention and small particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
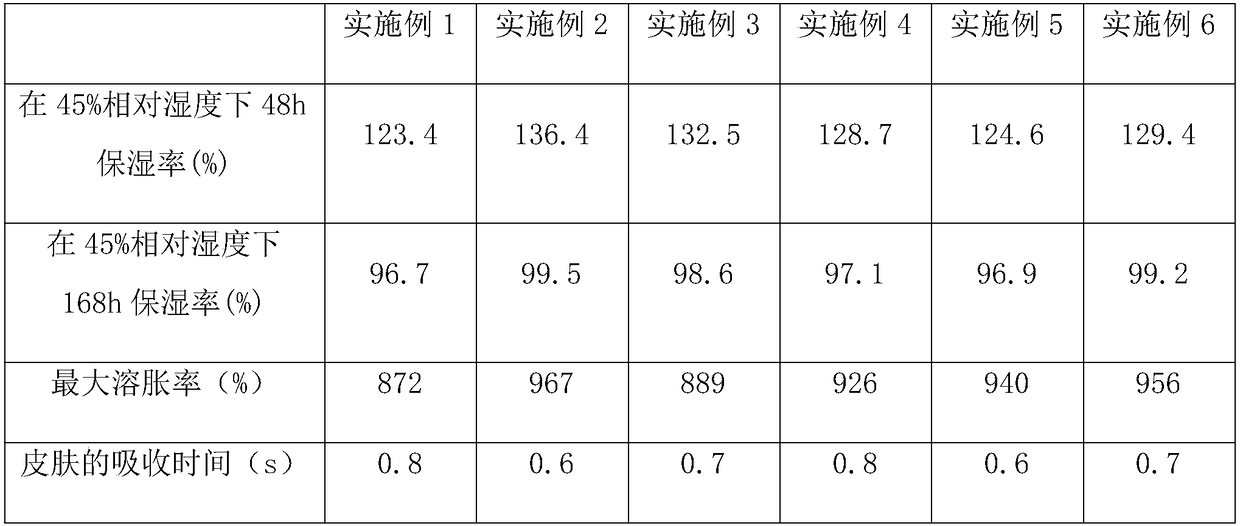
Examples
Embodiment 1
[0023] (1) In parts by weight, 1 part of sodium hyaluronate dry powder is added to an aqueous solution containing 0.001 part of hyaluronidase, and enzymatically hydrolyzed at 30°C for 20 minutes to obtain a small molecule of sodium hyaluronate of 2 μmoL / L solution, wherein the molecular weight of sodium hyaluronate is 100-250.
[0024] (2) Slowly add the sodium hyaluronate small molecule solution to the mixed oil phase at a rate of 5 μl / min, wherein the volume ratio of the sodium hyaluronate small molecule solution to the mixing tank is 1:40, and slowly stir at a rate of 25 r / min Finally, add a mixed cross-linking agent of calcium chloride and divinyl sulfone with a mass ratio of 1:0.6, the volume ratio of the cross-linking agent to the water phase is 3:1, and cross-link and solidify at 10°C for 4 hours to obtain micro-nano Grade Sodium Hyaluronate Microspheres.
[0025] (3) According to the mass ratio of sodium hyaluronate, schizophyllan and polyethylene glycol being 6:5:1, ...
Embodiment 2
[0027] (1) In parts by weight, 1 part of sodium hyaluronate dry powder is added to an aqueous solution containing 0.003 parts of hyaluronidase, and enzymatically hydrolyzed at 40°C for 30 minutes to obtain a small molecule of sodium hyaluronate of 5 μmoL / L solution, wherein the molecular weight of sodium hyaluronate is 100-250.
[0028] (2) Slowly add the sodium hyaluronate small molecule solution to the mixed oil phase at a rate of 10 μl / min, wherein the volume ratio of the sodium hyaluronate small molecule solution to the mixing tank is 1:50, and slowly stir at a rate of 80 r / min Finally, add a mixed cross-linking agent of calcium chloride and divinyl sulfone with a mass ratio of 1:0.8, the volume ratio of the cross-linking agent to the water phase is 4:1, and cross-link and solidify at 40°C for 6 hours to obtain micro-nano Grade Sodium Hyaluronate Microspheres.
[0029] (3) According to the mass ratio of sodium hyaluronate, schizophyllan and polyethylene glycol being 8:7:2...
Embodiment 3
[0031] (1) In parts by weight, 1 part of sodium hyaluronate dry powder is added to an aqueous solution containing 0.002 parts of hyaluronidase, and enzymatically hydrolyzed at 35°C for 23 minutes to obtain a small molecule of sodium hyaluronate of 3 μmoL / L solution, wherein the molecular weight of sodium hyaluronate is 100-250.
[0032] (2) Slowly add the sodium hyaluronate small molecule solution to the mixed oil phase at a rate of 8 μl / min, wherein the volume ratio of the sodium hyaluronate small molecule solution to the mixing tank is 1:45, and slowly stir at a rate of 50 r / min Finally, add a mixed cross-linking agent of calcium chloride and divinyl sulfone with a mass ratio of 1:0.7, the volume ratio of the cross-linking agent to the water phase is 3.5:1, and cross-link and solidify at 20°C for 4.5h to obtain micro Nanoscale sodium hyaluronate microspheres.
[0033] (3) According to the mass ratio of sodium hyaluronate, schizophyllan and polyethylene glycol of 7:6:1.2, sodi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com