Positive electrode active material for nonaqueous electrolyte secondary battery, method for producing same, and nonaqueous electrolyte secondary battery using same
A positive electrode active material and non-aqueous electrolyte technology, which is applied in the field of non-aqueous electrolyte secondary batteries, can solve the problems of electrode density decrease, damage performance, battery performance decrease, etc., and achieve the effect of less deterioration and stable charge and discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] An aqueous sodium hydroxide solution having a pH of 12.0 was prepared in a reactor equipped with a blade stirrer. Aqueous ammonia solution was dropped thereinto so that the ammonia concentration became 0.80 mol / l. A mixed aqueous solution of nickel sulfate, cobalt sulfate, and manganese sulfate was continuously supplied to the reactor at a composition ratio of Ni / Co / Mn=0.8 / 0.1 / 0.1. During this period, the aqueous sodium hydroxide solution and the aqueous ammonia solution were continuously supplied so that the pH value of the reaction solution reached 12 and the ammonia concentration reached 0.8 mol / l, and the reaction was continued until the target average secondary particle size was reached. During this period, spherical composite transition metal precipitates were obtained by applying mechanical shear force to the suspension.
[0080] After the reaction, the taken-out suspension was washed with water by a filter press, and then dried at 150° C. for 12 hours to obtain...
Embodiment 2
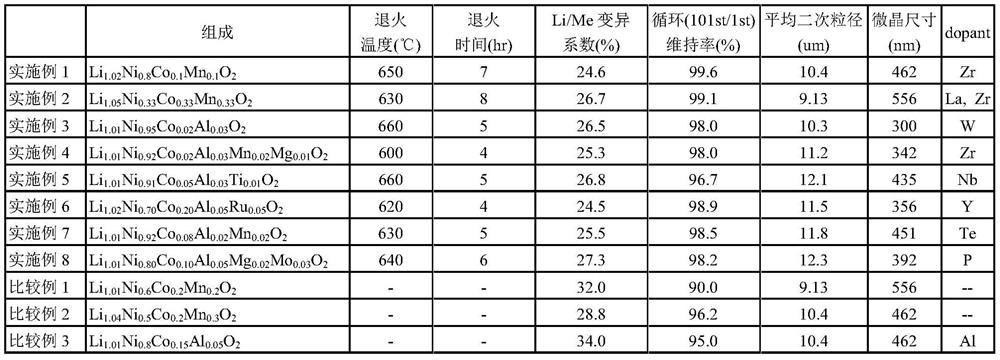
[0087] Change the mixing ratio of each compound so that the composition ratio of the precursor reaches Ni / Co / Mn=1.0 / 1.0 / 1.0, and adjust the ratio of Li to the metal constituting the precursor to the ratio shown in Table 1. Li raw material, Precursor, ZrO 2 , and La 2 o 3 The mixture was calcined at 850° C. for 10 hours in an oxidizing atmosphere, and then heat-treated at 630° C. for 8 hours in an air atmosphere as an annealing treatment. The calcined product after the annealing treatment was crushed to obtain positive electrode active material powder. Except for this, a positive electrode active material was obtained in the same manner as in Example 1.
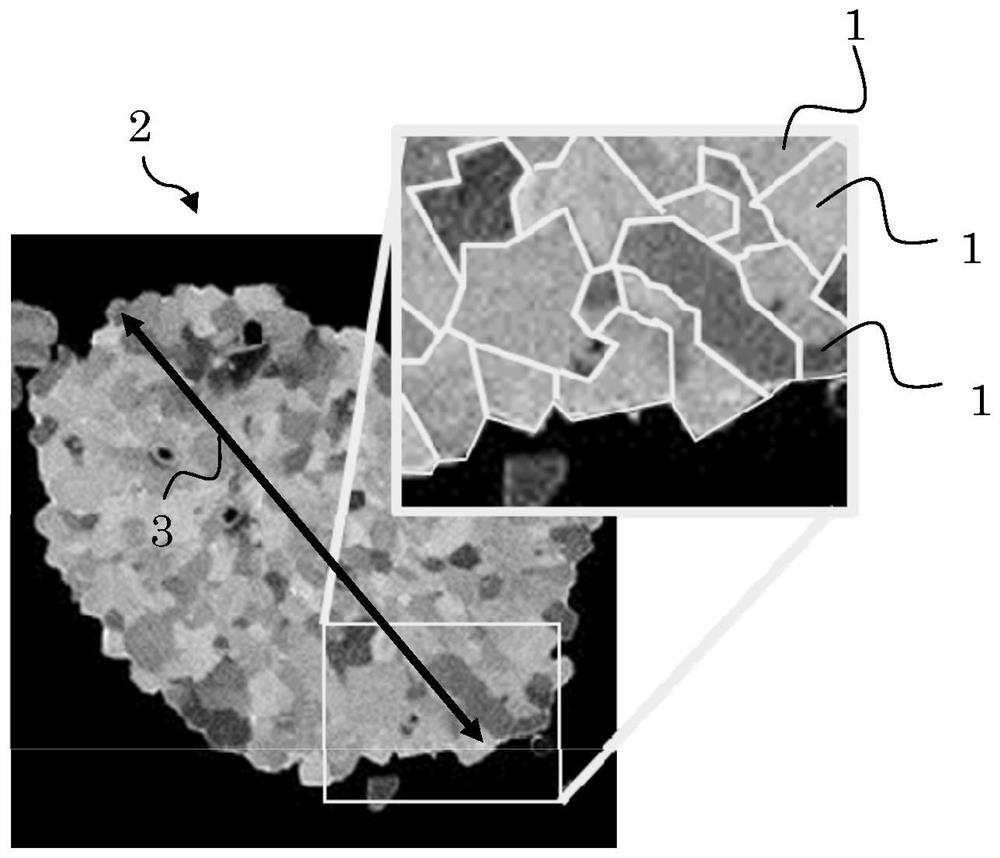
[0088] The variation coefficient of Li / M including crystals and grain boundaries was 26.7% after elemental distribution analysis using NanoSIMS in the cross section of the grains. In addition, it was confirmed that Zr coexists at grain boundaries where the Li concentration is high.
[0089] As an auxiliary measurement, hi...
Embodiment 3
[0092] An aqueous sodium hydroxide solution having a pH of 12.0 was prepared in a reactor equipped with a blade stirrer. Aqueous ammonia solution was dropped thereinto so that the ammonia concentration became 0.80 mol / l. A mixed aqueous solution of nickel sulfate, cobalt sulfate, and sodium aluminate was continuously supplied to the reactor. During this period, the aqueous sodium hydroxide solution and the aqueous ammonia solution were continuously supplied so that the pH value of the reaction solution reached 12 and the ammonia concentration reached 0.8 mol / l, and the reaction was continued until the target average secondary particle size was reached. During this period, spherical composite transition metal precipitates were obtained by applying mechanical shear force to the suspension.
[0093] After the reaction, the taken-out suspension was washed with water by a filter press, and then dried at 150° C. for 12 hours to obtain nickel-cobalt-aluminum compound particles (nick...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com