The preparation method of sodium vanadium fluorophosphate
The technology of sodium vanadium fluorophosphate and phosphoric acid is applied in the field of preparation of spherical sodium vanadium fluorophosphate, which can solve the problems of poor electrochemical performance of materials, serious agglomeration of material particles, easy loss of fluorine elements, etc. The effect of not easy to agglomerate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Dissolve ammonium dihydrogen phosphate and ammonium metavanadate at a molar ratio of 1:1 in deionized water, and then add oxalic acid 3 times the theoretical molar amount required for vanadium to be oxidized to trivalent vanadium, and control the total concentration to 0.5 mol / L, stirred at room temperature for 0.5h; the solution was subjected to spray pyrolysis at 600°C under an argon atmosphere (injection rate was 60ml / h, air intake volume was 3L / min), and vanadium phosphate powder was collected;
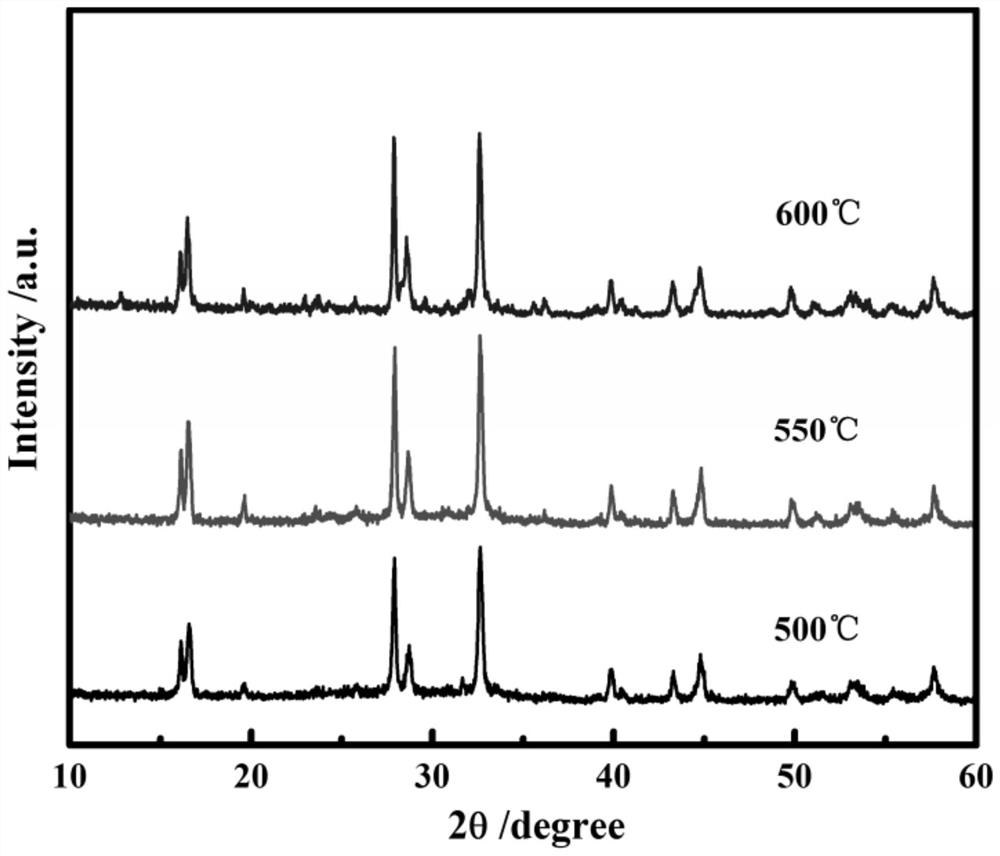
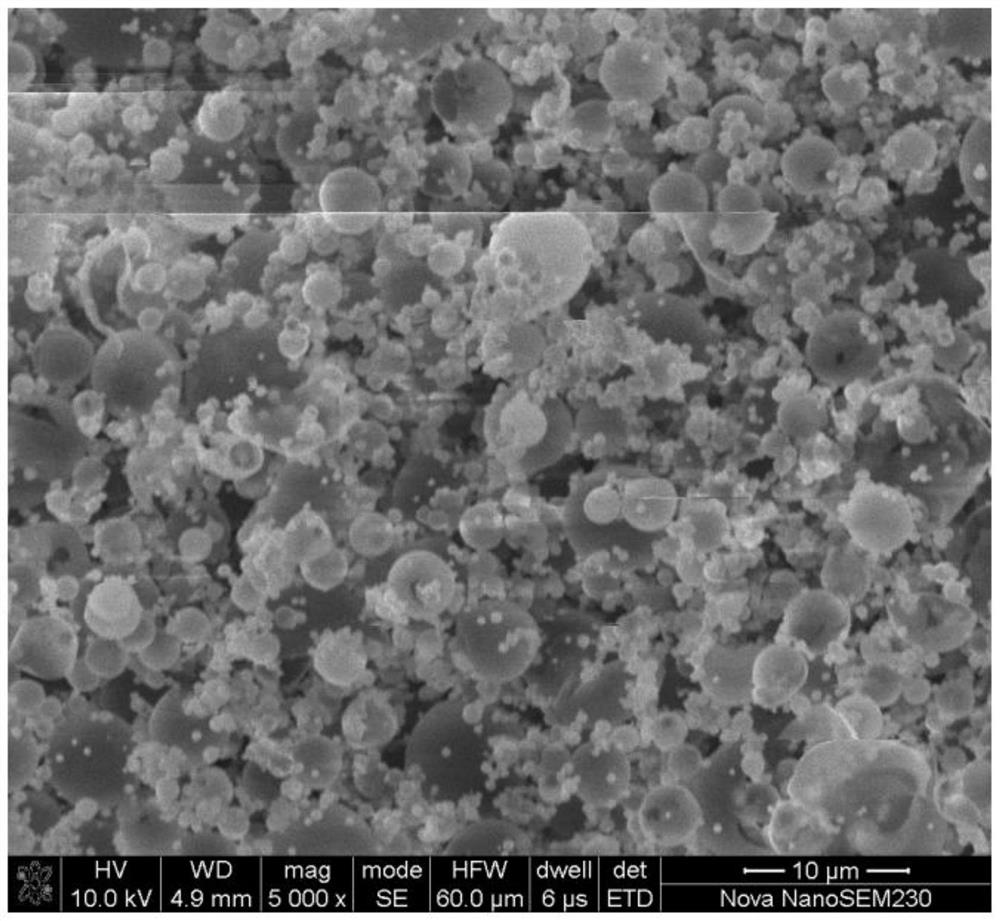
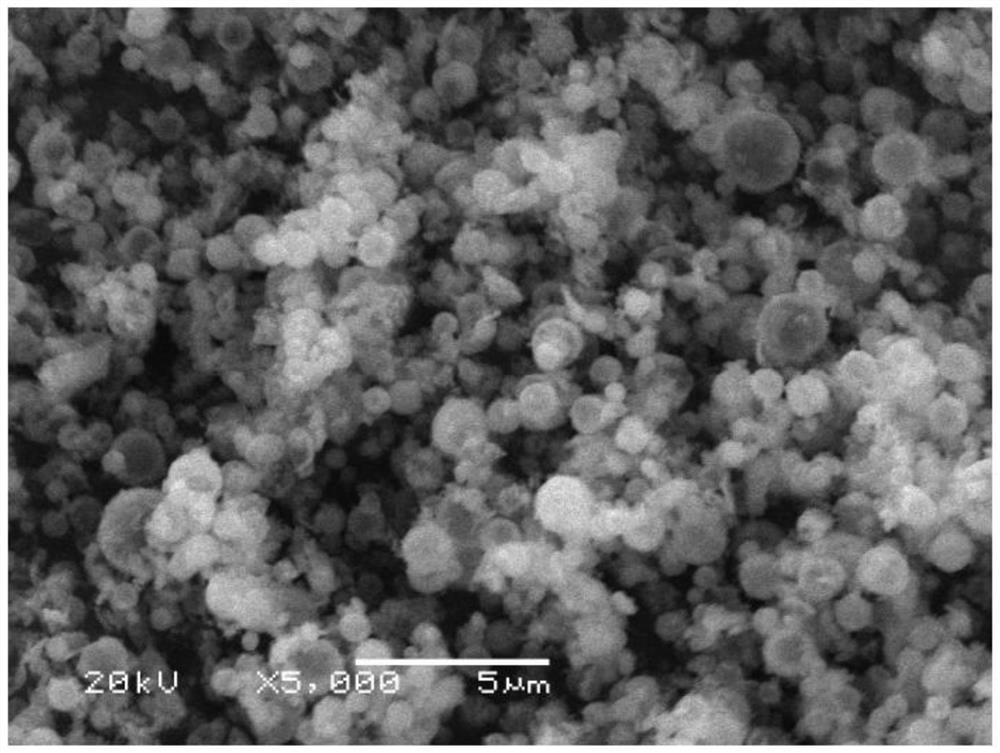
[0049] Mix the collected vanadium phosphate and sodium fluoride at a molar ratio of 1:1 and manually grind them for 1 hour, and then incubate at 500°C, 550°C, and 600°C for 4 hours in an argon atmosphere to obtain NaVPO with a spherical structure. 4 F. The obtained product was packed into a button battery to measure its charge-discharge specific capacity and rate performance, and the charge-discharge was carried out at different rates, and the first-time discharge specific...
Embodiment 2
[0053] Dissolve ammonium dihydrogen phosphate and ammonium metavanadate in deionized water at a molar ratio of 1:1, and then add oxalic acid according to 3 times the theoretical molar amount required for vanadium to be oxidized to trivalent vanadium, and control the total concentration to 0.5 mol / L, stirred at room temperature for 0.5h; the solution was subjected to spray pyrolysis at 600°C under an argon atmosphere (injection rate was 100ml / h, air intake volume was 2L / min), and vanadium phosphate powder was collected;
[0054] The collected vanadium phosphate and sodium fluoride were mixed and mechanically ground at a molar ratio of 1:1 for 0.5 h, and then placed in an argon atmosphere at 550 ° C for 4, 6, and 8 h to obtain NaVPO with a spherical structure. 4 F. The obtained product was assembled into a button battery to measure its charge-discharge specific capacity and rate performance, and the charge-discharge was carried out at different rates, and its first-time dischar...
Embodiment 3
[0058] Dissolve triammonium phosphate and vanadium pentoxide in deionized water at a molar ratio of 1:1, then add adipic acid according to 1 times the theoretical molar amount required for vanadium to be oxidized to trivalent vanadium, and control the total concentration to 0.1 mol / L, stirred at room temperature for 1 h; the solution was subjected to spray pyrolysis at 500°C under an argon atmosphere (injection rate was 110ml / h, air intake volume was 6L / min), and vanadium phosphate powder was collected;
[0059] Mix the collected vanadium phosphate, ammonium fluoride and sodium acetate according to the molar ratio of 1:1:1 and manually grind them for 2 hours, then place them in a nitrogen atmosphere at 650°C for 2 hours to obtain NaVP with a spherical structure. 4 F. Pack the obtained product into a button battery to measure its charge-discharge specific capacity and rate performance, and the discharge specific capacity at 0.1C, 1C, 2C, and 5C rates is 130, 123, 118, and 112mA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com