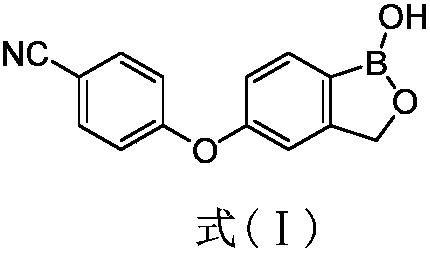
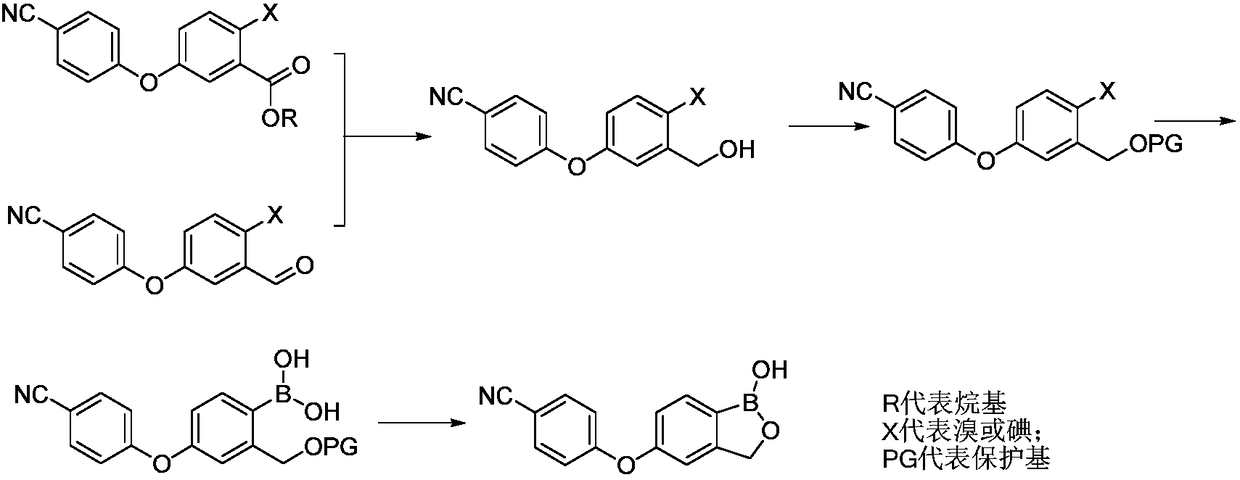
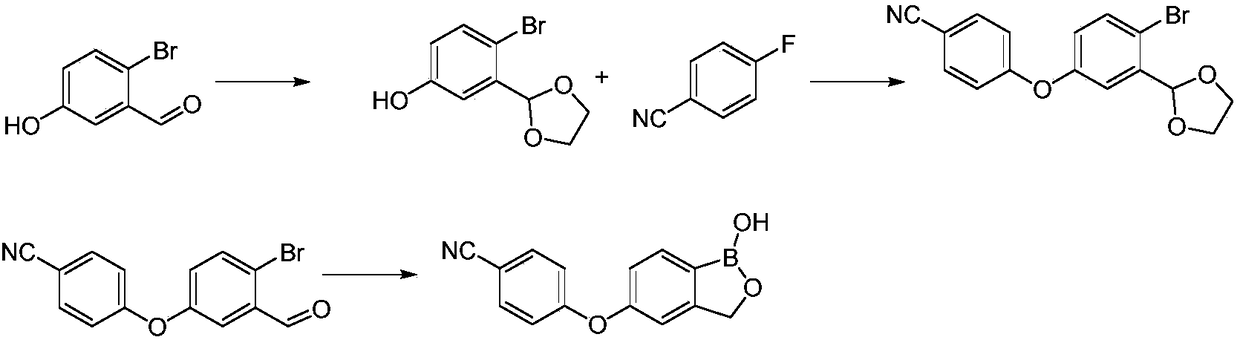
Preparation method of boracic micromolecule
A technology of small molecules and borate esters, which is applied in the field of preparation of boron-containing small molecule crisborole, can solve the problems of high cost and low yield, and achieve the effects of low cost, simple operation, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Embodiment 1 Synthetic 2-bromo-5-hydroxybenzyl alcohol (III)
[0059]
[0060] Add 40g (0.20mol) 2-bromo-5-hydroxybenzaldehyde (II) and 400mL MeOH to the reaction flask, and stir evenly; add 5.6g (0.15mol) sodium borohydride in batches under an ice bath, and keep the reaction for 0.5h~ 1.5h. TLC detected that the reaction of the raw material was basically complete, quenched by adding 100mL of water, extracted with 400mL of ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain an off-white solid, added 100mL of dichloromethane to beat at room temperature for 0.5h, filtered, and the filter cake was air-dried Obtained 37.2g white solid namely 2-bromo-5-hydroxybenzyl alcohol (III), molar yield 91.6%, MS (m / z): 200.9 [M-H] - .
Embodiment 2
[0061] Example 2 Synthesis of 4-benzyloxy-2-benzyloxymethyl-1-bromobenzene (IV-1)
[0062]
[0063] Add 34g (0.17mol) 2-bromo-5-hydroxybenzyl alcohol (III), 50mL N,N-dimethylformamide solution to the reaction flask, add 12.3g (60%, 0.51mol) in batches under ice-cooling Sodium hydrogen, keep warm at 0~5℃ for 0.5h~1.5h after adding, slowly add 73.5g benzyl bromide (0.43mol) dropwise, keep warm for 0.5h~1.5h after dropping, add 100mL water to quench, 200mL acetic acid Extract with ethyl ester, dry over anhydrous sodium sulfate, concentrate under reduced pressure, remove benzyl bromide through column chromatography (PE:EA=80:1~40:1), and obtain 59.4 g of colorless oily liquid which is 4-benzyloxy -2-Benzyloxymethyl-1-bromobenzene (IV-1), the molar yield is 91.2%. 1 H-NMR (400Hz, CDCl 3 ) δ (ppm) 4.59 (s, 2H), 4.63 (s, 2H), 5.06 (s, 2H), 6.79 (dd, J = 4Hz, 1H), 7.20 (d, J = 4Hz, 1H), 7.38 ( m, 11H).
Embodiment 3
[0064] Example 3 Synthesis of 4-benzyloxy-2-benzyloxymethylphenylboronic acid (V-1)
[0065]
[0066] Add 59g (0.15mol) 4-benzyloxy-2-benzyloxymethyl-1-bromobenzene (IV-1) and 580ml tetrahydrofuran solution into the reaction flask, stir evenly, protect with nitrogen, cool to -78°C, Add dropwise 72mL of 2.5mol / L n-butyllithium n-hexane solution, keep warm for 0.5h, add dropwise 37.6g (0.2mol) triisopropyl borate, keep warm at -65~-78℃ during the dropping process, keep warm for 0.5h , TLC detected that the reaction of the raw material was complete, raised to room temperature, quenched with saturated ammonium chloride solution, extracted with 500ml ethyl acetate, dried over anhydrous sodium sulfate, concentrated under reduced pressure to obtain a yellow oily liquid, added 450mL of n-heptane, and stirred at room temperature for 0.5h , precipitated off-white solid, filtered, and dried to obtain 43.4 g of white solid, namely 4-benzyloxy-2-benzyloxymethylphenylboronic acid (V-1), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com