A class of (z)-2-thio-β-aminocrotononitrile compounds and their electrochemical preparation methods
An aminocrotononitrile and compound technology, which is applied in the field of electrochemical organic synthesis, can solve the problem that compound synthesis work has not been reported, and achieve the effects of simple and efficient reaction system, high yield and wide application range.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] With metal platinum sheet as anode and platinum wire as cathode, add 0.5mmol tetra-n-butylammonium perchlorate, 0.05mmol tartaric acid, 0.1mmol (1,2-bis(diphenylphosphino)ethyl alkane), 0.25mmol potassium iodide, 0.5mmol p-fluorothiophenol, 5mL acetonitrile, a magnetic stirrer, cover the lid, turn on the power, adjust the current to 10mA, and electrolyze for 6h at room temperature. After the reaction, the reaction solution was extracted with ethyl acetate, and the corresponding product 3a was obtained after separation and purification, and the yield of the product 3a was 91%. The reaction scheme of the present embodiment is as follows:
[0042]
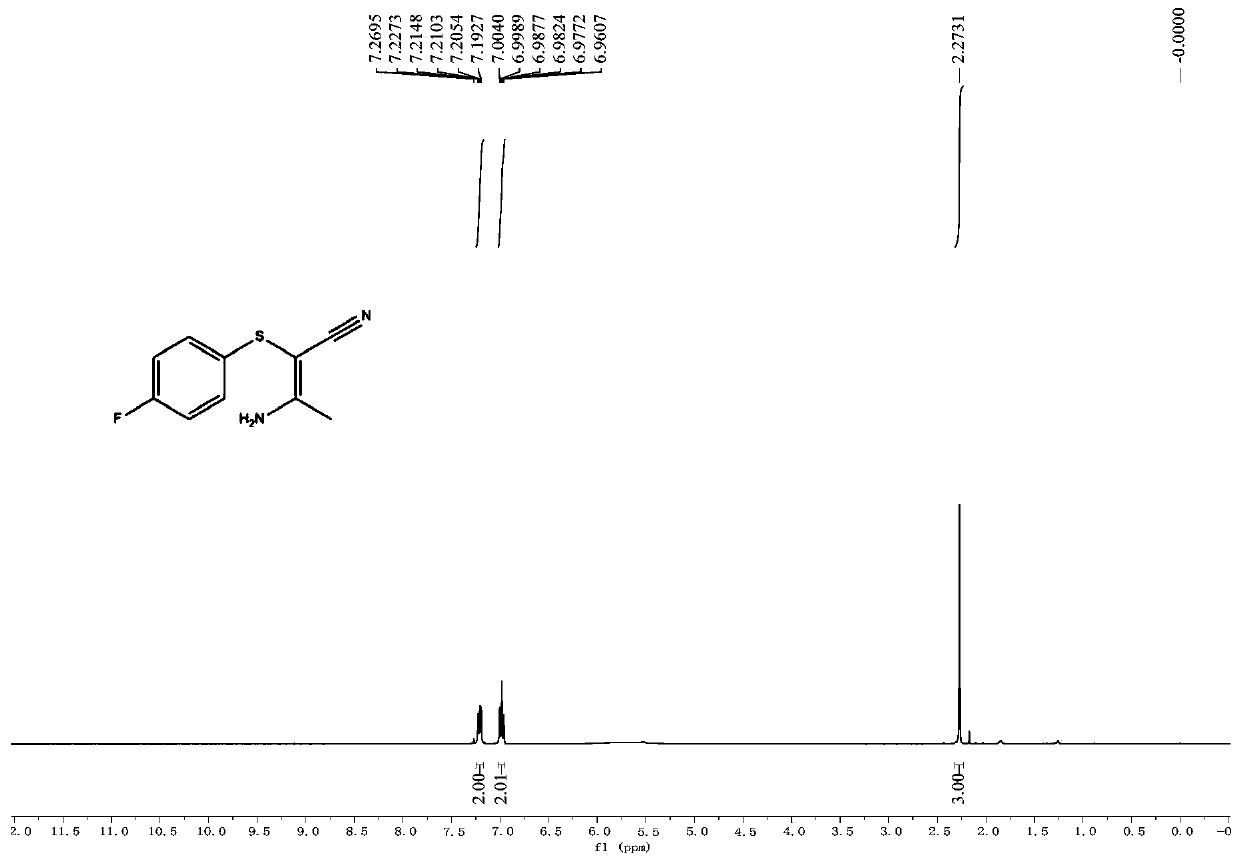
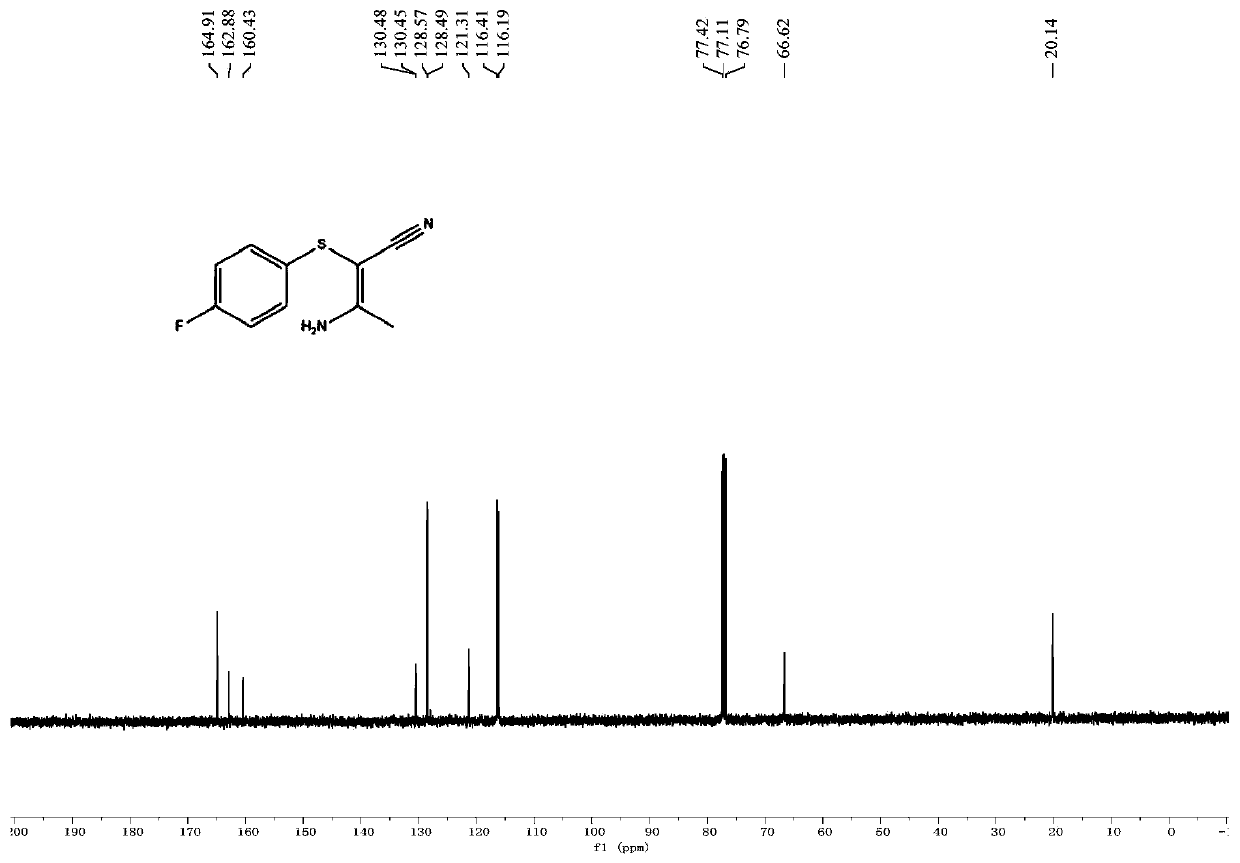
[0043] The proton nuclear magnetic resonance spectrum of the product of this embodiment is as follows: figure 1 Shown: 1 H NMR (400MHz, CDCl 3 ):δ7.24–7.19(m,2H),7.00–6.96(m,2H),5.66(br s,2H),2.27(s,3H); figure 2 Shown: 13 C NMR (100MHz, CDCl 3 ):δ164.9,161.7(d,J C-F =246.0Hz), 130.4(d, J C-F =3.2Hz), 128.5(d, J C-...
Embodiment 2
[0045] With metal platinum mesh as anode and platinum sheet as cathode, add 0.5mmol tetra-n-butylammonium perchlorate, 0.05mmol citric acid, 0.1mmol bis(diphenylphosphino)methane, 0.3mmol iodine Ammonium chloride, 0.5mmol p-fluorothiophenol, 5mL acetonitrile, a magnetic stirrer, cover the lid, turn on the power, adjust the current to 15mA, and electrolyze at room temperature for 8h. After the reaction, the reaction liquid was extracted with ethyl acetate, and the corresponding product 3a was obtained after separation and purification, and the yield of the product 3a was 85%. The reaction scheme of the present embodiment is as follows:
[0046]
Embodiment 3
[0048] With metal platinum mesh as anode and platinum wire as cathode, add 0.5mmol tetra-n-butylammonium perchlorate, 0.05mmol citric acid, 0.1mmol 1,2-bis(diphenylphosphino)ethyl Alkanes, 0.35mmol potassium iodide, 0.5mmol p-fluorothiophenol, 5mL acetonitrile, a magnetic stirrer, cover the lid, turn on the power, adjust the current to 10mA, and electrolyze for 4h at room temperature. After the reaction, the reaction solution was extracted with ethyl acetate, and the corresponding product 3a was obtained after separation and purification, and the yield of the product 3a was 92%. The reaction scheme of the present embodiment is as follows:
[0049]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com