Reorganized corynebacterium glutamicum producing chondroitin and application of reorganized corynebacterium glutamicum
A technology of Corynebacterium glutamicum and chondroitin synthase, which is applied in the field of bioengineering, can solve the problems of increasing downstream product processing, difficulty in purification, lack of key enzyme transformation for chondroitin synthesis, and low chondroitin yield, and avoids fructosaccharification. Effects of modification, improved association, and low cost of cultivation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1: P trc -kfoA-kfoC integrated into the C. glutamicum genome
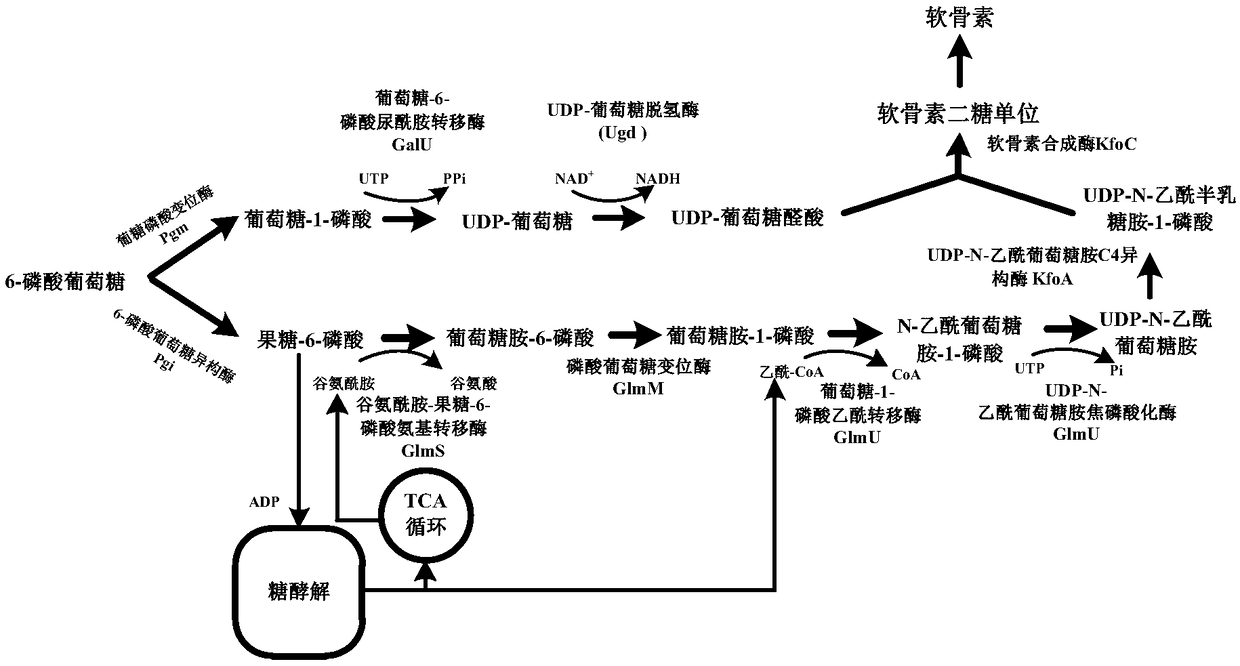
[0018] Ptrc-F and Ptrc-R were used as primers, and pEC-XK99E was used as template to amplify P trc , with kfoA-F and kfoA-R as primers, kfoA was amplified with the Escherichia coli K4 genome as a template; with kfoC-F and kfoC-R as primers, kfoC was amplified with the Escherichia coli K4 genome as a template; the obtained P trc , kfoA and kfoC used Ptrc-F and kfoC-R as primers for overlap extension PCR to obtain P trc -kfoA-kfoC expression cassette. With glgA up-F and glgA down-R as primers, with P trc -kfoA-kfoC and Corynebacterium glutamicum glgA gene upper and lower 500bp homology arms (two 500bp homology arms respectively through primers glgA up-F and glgAup-R, glgA down-F and glgA down-R with glutamine Acid Corynebacterium ATCC13032 genome as a template for PCR) as a template for overlapping extension PCR again to obtain H1-P trc-kfoA-kfoC-H2.
[0019] H1-P trc -kfoA-kfoC-H2 was ligated i...
Embodiment 2
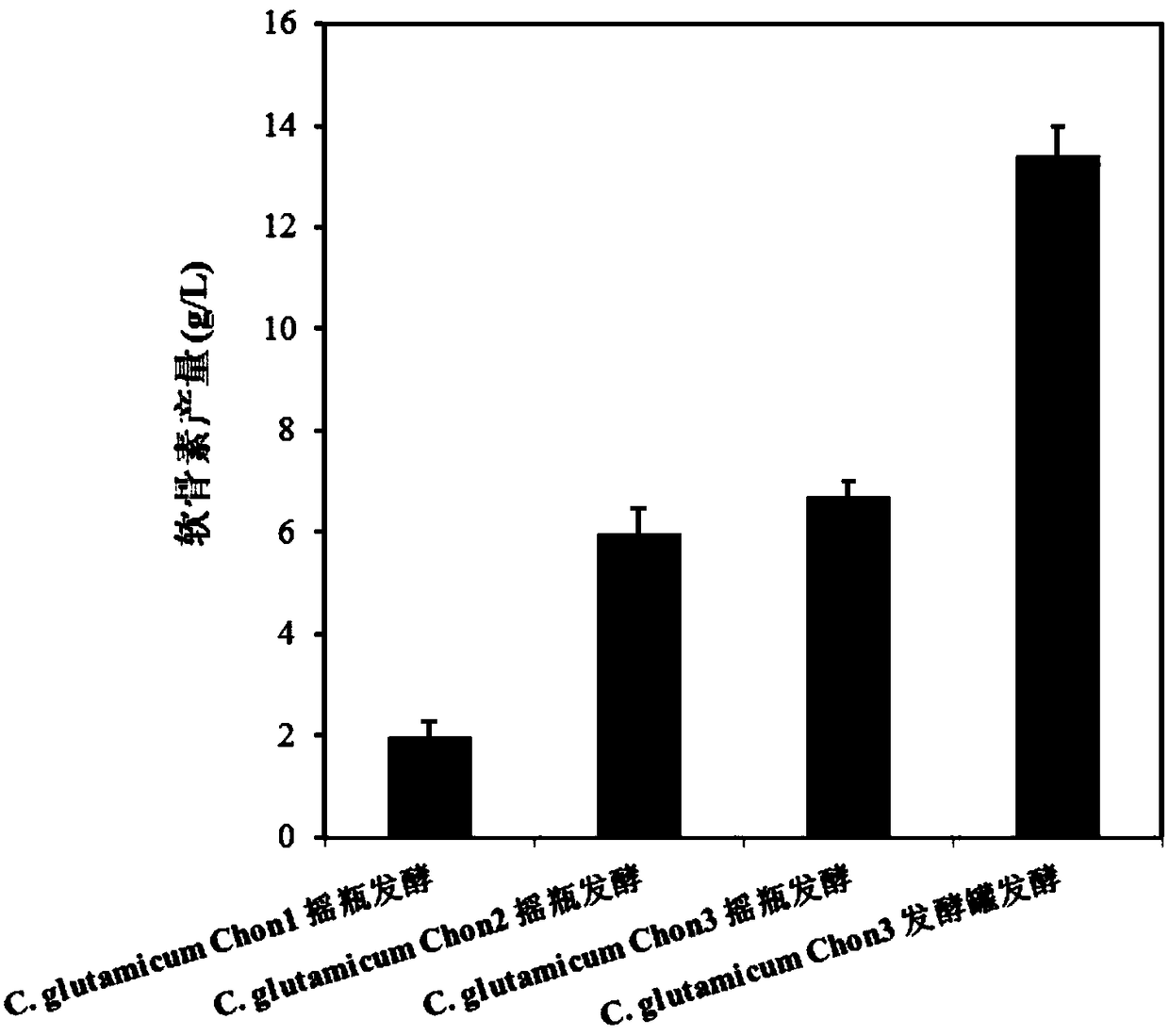
[0020] Embodiment 2: the construction of recombinant bacteria C.glutamicum Chon1
[0021] Corynebacterium glutamicum ATCC 13032 was inoculated in 5 mL of BHIS liquid medium, cultured at 30°C and 200 rpm for 24 hours, the cells were collected, and genomic DNA was extracted using a cell genome extraction kit. Step 1: Construction of pXMJ19-glmU-galU. Primers Ppyc-F / Ppyc-R, glmU-F / glmU-R, galU-F / galU-R were designed, using the extracted C. glutamicum ATCC 13032 genomic DNA as a template, using standard PCR amplification system and procedures to amplify Add Ppyc, glmU, galU genes. The amplified glmU and galU were used as templates and glmU-F and galU-R were used as primers to perform overlap extension PCR to obtain connected glmU-galU fragments. Using pXMJ19-F / pXMJ19-R as primers to amplify the linear pXMJ19 plasmid with deletion of Ptac and lacIQ regions. The glmU-galU fragment and the linearized plasmid pXMJ19 were subjected to one-step cloning and splicing reactions, respect...
Embodiment 3
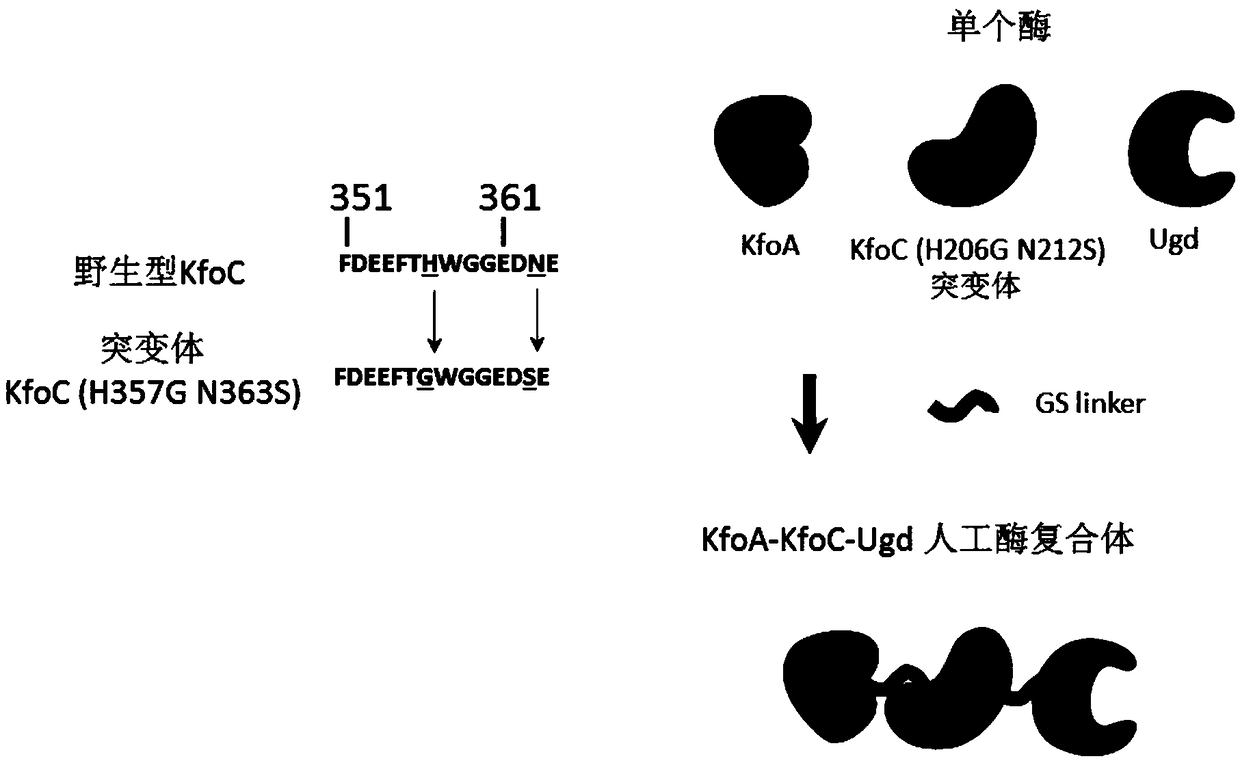
[0025] Example 3: Construction and screening of KfoC (H357G / N363S) mutants
[0026] According to the operation manual of the kit, using kfoC-F and kfoC-R as primers, use Diversify TM PCR RandomMutagenesis Kit PCR Random Mutagenesis Kit performs random mutation on the kfoC gene. According to the description in Example 1, P trc Promoter and kfoA gene assembly to form P trc -kfoA-kfoC (random mutant) expression cassette, and according to the description in Example 1, the P trc -kfoA-kfoC (random mutant) expression cassette integrated into the C. glutamicum genome. Contains P trc The recombinant Corynebacterium glutamicum single colony library of -kfoA-kfoC (random mutant) was fermented in a 96-well plate. After the glucose was exhausted (after 40 hours), the bacterial cells were collected by centrifugation in a 96-well plate, and the bacterial cells were washed twice with deionized water. After the bacteria were diluted by appropriate multiples, the content of chondroitin w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com