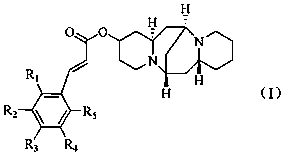
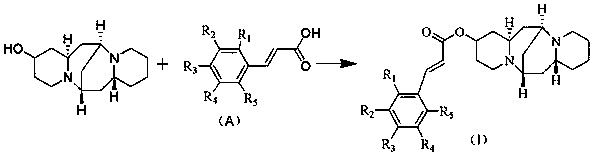
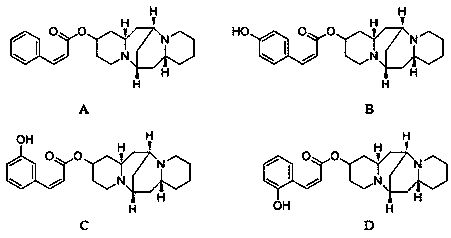
13-hydroxyl cytisine cinnamate ester compound having antitumor activity and preparation method thereof
A compound and hydroxyl technology, applied in the field of 13-hydroxycytisine cinnamate compounds and their preparation, can solve problems such as multi-drug resistance and treatment failure, and achieve enhanced drug efficacy, full utilization, and low production costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (Z)-(6S,7aR,13S,14aR)-tetradecahydro-6,13-methanodipyrido[1,2-a:1',2'-e][1,5]diazocin-2-yl 3-phenylacrylate ( Preparation of Compound A)
[0032] Put 25.02mg (0.10mmol) of 13-hydroxycytisine and 19.25mg (0.13mmol) of cinnamic acid in a reaction vessel, add 50mL of toluene to fully dissolve, add 2.46mg (0.02mmol) of 4-DMAP, 2.06mg ( 0.01mmol) DCC, reacted at 80°C for 8 hours, evaporated to dryness under reduced pressure, added ethyl acetate to dissolve the organic matter, washed the organic layer with water, sodium bicarbonate solution and brine, and dried, and recovered the solvent by rotary evaporation under reduced pressure. The concentrated solution was cooled and crystallized, and 29.00 mg of the target compound A was obtained by suction filtration, with a yield of 76.26%.
[0033]
[0034] 1 H-NMR (400MHz, DMSO- d 6 )δ(ppm): 7.60(2H,d), 7.32-7.40(4H,m), 6.59 (1H,d), 3.92(1H,s), 2.72-3.17 (4H,m), 2.64-2.72(4H ,m), 1.76-1.93 (4H,m), 1.48-1.69 (4H,m), 1.45-1.4...
Embodiment 2
[0036] (Z)-(6S,7aR,13S,14aR)-tetradecahydro-6,13-methanodipyrido[1,2-a:1',2'-e][1,5]diazocin-2-yl 3-(4 Preparation of -hydroxyphenyl)acrylate (compound B)
[0037] Put 25.02mg (0.10mmol) of 13-hydroxycytisine and 16.40mg (0.10mmol) of 4-hydroxycinnamic acid in a reaction vessel, add 50mL of acetonitrile to fully dissolve, then add 2.46 mg (0.02mmol) of 4-DMAP, 2.06mg (0.01mmol) DCC, reacted at 80°C for 12 hours, evaporated to dryness under reduced pressure, added ethyl acetate to dissolve the organic matter, washed the organic layer with water, sodium bicarbonate solution and brine, and dried, and rotated under reduced pressure The solvent was recovered by evaporation, the concentrated solution was cooled and crystallized, and 27.94 mg of compound B of the present invention was obtained by suction filtration, with a yield of 70.52%.
[0038]
[0039] 1 H-NMR (400MHz, DMSO- d 6 )δ(ppm):7.48(2H,d), 7.37(1H,d), 6.56 (2H,d), 5.90(1H,d), 5.28(1H,s), 5.19(1H,m), 2.58( 1H,m),...
Embodiment 3
[0041] (Z)-(6S,7aR,13S,14aR)-tetradecahydro-6,13-methanodipyrido[1,2-a:1',2'-e][1,5]diazocin-2-yl 3-(3 Preparation of -hydroxyphenyl)acrylate (compound C)
[0042]Put 25.02mg (0.10mmol) of 13-hydroxycytisine and 16.40mg (0.10mmol) of 3-hydroxycinnamic acid in a reaction vessel, add 50mL of toluene to fully dissolve, then add 2.46 mg (0.02mmol) of 4-DMAP, 1.92 mg (0.01 mmol) of EDC, reacted at 80°C for 4 hours, evaporated to dryness under reduced pressure, added ethyl acetate to dissolve the organic matter, washed the organic layer with water, sodium bicarbonate solution and brine, dried, and rotated under reduced pressure The solvent was recovered by evaporation, the concentrated solution was cooled and crystallized, and 27.13 mg of compound C of the present invention was obtained by suction filtration, with a yield of 68.48%.
[0043]
[0044] 1 H-NMR (400MHz, DMSO- d 6 )δ(ppm): 7.48(1H,t), 7.40(1H,d), 7.09 (1H,d),6.75 (1H,d), 6.62 (1H,s), 5.87(1H,d), 5.16( 1H,s), 5.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com