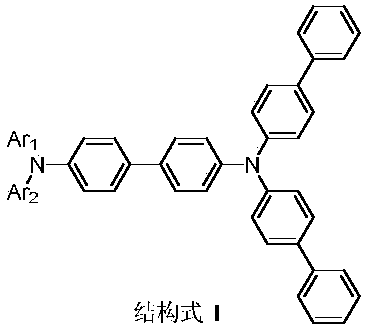
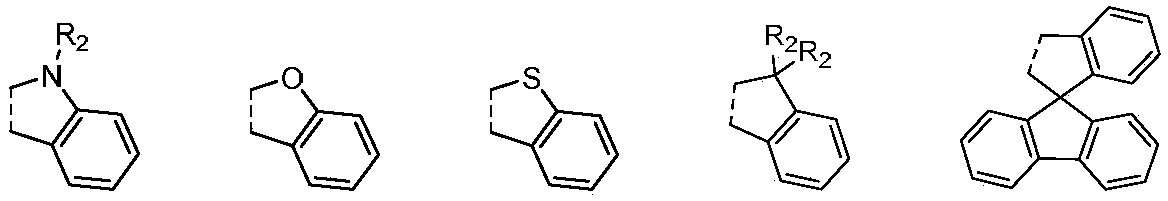
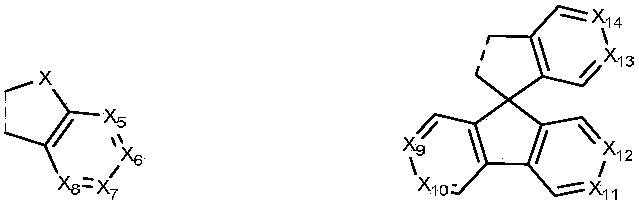Benzidine derivative and organic light-emitting diode thereof
A technology of electroluminescent devices and derivatives, applied in the direction of electric solid state devices, electrical components, organic chemistry, etc., can solve the problems of unsatisfactory luminescence characteristics, improve the glass transition temperature and thermal stability, and achieve long-term use Effects of Lifetime, High Luminous Efficiency and Luminous Brightness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0080] Preparation of Compound Sub I-1:
[0081]
[0082] Under argon protection, bis(4-biphenyl)amine (10g, 31mmol), 4-bromo-4-iodobiphenyl (11.1g, 31mmol), sodium tert-butoxide (3g, 31mmol), Bis(triphenylphosphine)palladium(II) dichloride (0.5g, 0.71mmol) and toluene (500ml) were reacted at 130°C for 24 hours. After cooling, water (1000ml) was added, the mixture was filtered, the filtrate was extracted with toluene, and the organic phase was dried over anhydrous magnesium sulfate. This was concentrated under reduced pressure, and the resulting crude product was column-purified, recrystallized from toluene, filtered, and dried to obtain an intermediate Sub I-1 (10.5 g, 61%).
Embodiment 1
[0084] Preparation of Compound Sub II-1:
[0085]
[0086] Under argon protection, 3-methoxy-2-naphthaleneboronic acid (21.2g, 105mmol), 1-bromo-3-fluoro-4-iodobenzene (30.0g, 100mmol), Pd(PPh 3 ) 4 (2.31g, 2mmol), toluene (300ml), aqueous sodium carbonate solution (2M, 150ml), and stirred at reflux for 8 hours. After the above reaction solution was cooled to room temperature, it was extracted with toluene, the organic phases were combined, and the organic phase was washed with saturated brine. After the organic phase was dried and concentrated, column chromatography was performed using silica gel as a stationary phase to obtain compound A1 (23.2 g, 70%).
[0087] Add compound A1 (23.2g, 70mmol) and dry dichloromethane (200ml) to the flask successively, and cool the reaction system to 0°C. Join BBr 3 (22.0g, 88mmol), then stirred at room temperature for 24 hours. After the reaction, the solution was cooled to -78°C, carefully deactivated with methanol, and then deactiv...
Embodiment 2
[0102] Preparation of Compound Sub III-2:
[0103]
[0104] Under argon protection, 3-bromo-9-ethylcarbazole (27.4 g, 100 mmol) was added to the flask, poured into an appropriate amount of anhydrous THF to dissolve, cooled to -78 ° C, and added dropwise n-butyllithium ( 7.7g, 120mmol), heat preservation reaction for 0.5 hours, quickly drop triisopropyl borate (28.2g, 150mmol), slowly warm up to room temperature, and react for 30min. After the reaction was completed, the reaction solution was poured into dilute hydrochloric acid aqueous solution, and a solid substance was precipitated and filtered, and the crude product was passed through a silica gel column to obtain compound C1 (23.8 g, 75%).
[0105] Under argon protection, compound C1 (40.7g, 128.1mmol), 2-nitroiodobenzene (26.6g, 106.8mmol), Na 2 CO 3 (34.0g, 320.4mmol), tetrakistriphenylphosphine palladium (6.17g, 5.3mmol), toluene (640ml) and ethanol (160ml), the reaction mixture was stirred at 90°C for 3 hours. Af...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com