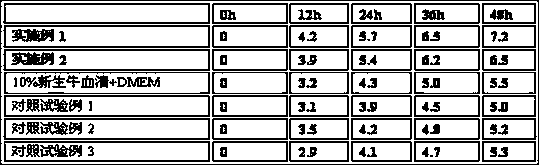
A kind of method that utilizes serum-free medium to produce pedv inactivated vaccine
A technology of serum-free culture medium and inactivated vaccine, applied in the field of PEDV inactivated vaccine production, can solve the problems of high cost of serum production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1: a kind of serum-free medium suitable for large-scale production of PEDV vaccine is characterized in that, described serum-free medium is made of basal medium, bovine serum albumin, cortisol, insulin, transferrin, glutathione It consists of peptide, trypsin, taurine and sodium selenite, wherein the weight ratio of taurine and sodium selenite is 2:1.
[0046] The basal medium IMDM (Giboc);
[0047] The content of the bovine serum albumin is 10mg / mL.
[0048] The content of the cortisol is 20 μg / mL.
[0049]The content of the insulin is 20 μg / mL.
[0050] The content of the transferrin is 15 μg / mL.
[0051] The content of glutathione is 3mM.
[0052] The content of the trypsin is 4 μg / mL.
[0053] The content of the taurine is 20 μg / mL.
[0054] The content of the sodium selenite is 10 μg / mL.
[0055] The preparation method of the serum-free medium that is suitable for large-scale production of PEDV vaccine of the present invention is: step 1, take by ...
Embodiment 2
[0056] Embodiment 2: a kind of serum-free medium suitable for large-scale production of PEDV vaccine is characterized in that, described serum-free medium is made of basal medium, bovine serum albumin, cortisol, insulin, transferrin, glutathione It consists of peptide, trypsin, taurine and sodium selenite, wherein the weight ratio of taurine and sodium selenite is 3:1.
[0057] The basal medium IMDM (Giboc);
[0058] The content of the bovine serum albumin is 10mg / mL.
[0059] The content of the cortisol is 20 μg / mL.
[0060] The content of the insulin is 20 μg / mL.
[0061] The content of the transferrin is 15 μg / mL.
[0062] The content of glutathione is 3mM.
[0063] The content of the trypsin is 4 μg / mL.
[0064] The content of the taurine is 30 μg / mL.
[0065] The content of the sodium selenite is 10 μg / mL.
[0066] The preparation method of the serum-free medium that is suitable for large-scale production of PEDV vaccine of the present invention is: step 1, take by...
Embodiment 3
[0067] Embodiment 3: PEDV small-scale proliferation test
[0068] Test strain: PEDV SX-YL strain, obtained from Northwest Agriculture and Forestry University, which was isolated from a large-scale farm in Yangling, Shaanxi.
[0069] Experimental cells: Vero cell line, a gift from the Veterinary Immunology Laboratory, School of Veterinary Medicine, Northwest A&F University.
[0070] experiment method:
[0071] (1) Recovery of Vero cell lines:
[0072] (1) Quickly put Vero cells taken out of liquid nitrogen into a 37°C water bath, shake gently to thaw quickly;
[0073] (2) Centrifuge at 300 rpm for 10 min;
[0074] (3) Discard the supernatant, add 37°C pre-warmed cell culture medium (containing 5% FBS DMEM), and gently blow off the Vero cells;
[0075] (4) Add the mixed cell suspension into a sterile T25 bottle containing 5m L5% FBS DMEM cell culture medium, and culture it in a cell culture incubator with suitable conditions;
[0076] (5) After two passages, add the vero ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com