Lysine decarboxylase mutant, encoding gene and expression thereof and application
A technology of lysine decarboxylase and mutants, which is applied in the field of lysine decarboxylase mutants, can solve the problem of weak tolerance of lysine decarboxylase, and achieves improved alkali resistance and catalytic performance, improved enzyme activity, The effect of alkali resistance and catalytic performance enhancement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The construction of embodiment 1 recombinant plasmid pETDuet-1-CadA
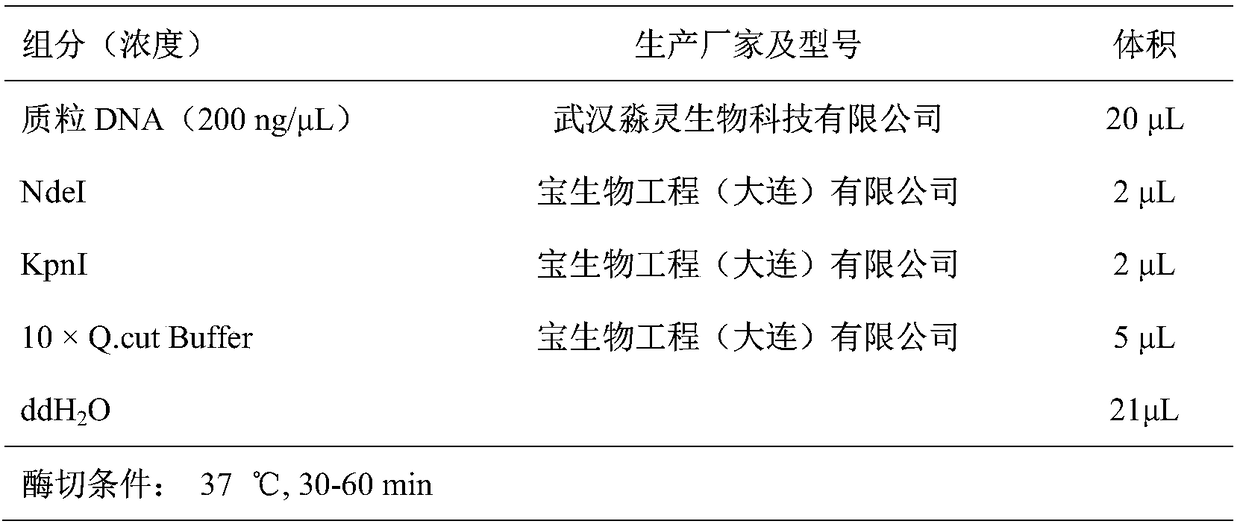
[0043] Using the genome of Escherichia coli MG1655 (purchased from Wuhan Miaoling Biotechnology Co., Ltd.) as a template, the primers at both ends of the CadA gene were used for PCR amplification to obtain the CadA fragment (see Table 1), and then cloned into the vector pETDuet-1 (purchased from Wuhan Miaoling Biotechnology Co., Ltd. Between the NdeI and KpnI restriction sites of Ling Biotechnology Co., Ltd.) (see Table 2 and Table 3), the recombinant plasmid pETDuet-1-CadA was obtained. The nucleotide sequence of the CadA gene in Escherichia coli MG1655 is shown in SEQ ID No:1, and the amino acid sequence of the inducible lysine decarboxylase encoded by it is shown in SEQ ID No:2.
[0044] Wherein, the primers at both ends are as follows:
[0045] Primer1-F: 5'-GGAATTCCATATGAACGTTATTGCAATATTG-3'
[0046] Primer2-R: 5'-GGGGTACCTTTATTTTTTGCTTTCTTCTTTC-3';
[0047] Table 1 PCR reaction system and rea...
Embodiment 2
[0053] Example 2 Construction of site-directed mutagenesis plasmid
[0054] By analyzing the tertiary structure of the lysine decarboxylase decamer, excluding the amino acids located in the decarboxylase monomer and the conserved amino acids, three pairs of amino acids were selected and mutated into cysteines with stronger disulfide bonds. Improve the binding force of disulfide covalent bonds.
[0055] Using the plasmid pETDuet-1-CadA prepared in Example 1 as a template, the site-directed mutation plasmid pETDuet-1-CadA-M1(M2 / M3) was obtained by two rounds of PCR (polymerase chain reaction) amplification in vitro: M1(L89C / L442C) is about to mutate the 89th leucine / 442nd leucine of lysine decarboxylase such as the amino acid sequence shown in SEQ ID No: 2 into cysteine respectively, and M2 (F102C / L547C) is about the amino acid sequence as shown in The 102nd phenylalanine / 547th leucine of the lysine decarboxylase shown in SEQ ID No: 2 are mutated into cysteine respectively...
Embodiment 3
[0085] Embodiment 3 Verification amplification of mutant expression vector
[0086] The PCR stock solution after excising the template DNA obtained in Example 2 was transformed into E.coli Trans1-T1 (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) competent cells, and coated with 100mg / L ampicillin-resistant On the LB plate, place the plate upside down in a 37°C incubator and incubate overnight. Pick several single colonies, extract the plasmid for sequencing, and after confirming that the mutation is correct, transform the correct plasmid into E.coli BL21 (DE3) (purchased from Beijing Quanshijin Biotechnology Co., Ltd.) Strain E. coli BL21(DE3) (pETDuet-1-CadA-M1(M2 / M3)) of lysine decarboxylase mutant with improved activity.
[0087] Among them, the formula of LB solid medium containing 100mg / L ampicillin is as follows: peptone 10g / L; yeast powder 5g / L; sodium chloride 5g / L; agar powder 25g / L; ampicillin 0.1g / L.
[0088] Wherein, the method for DNA conversion is:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com