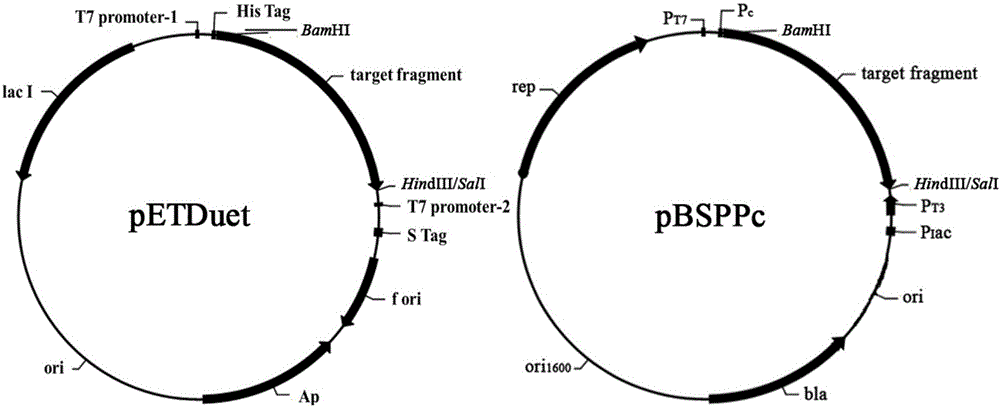
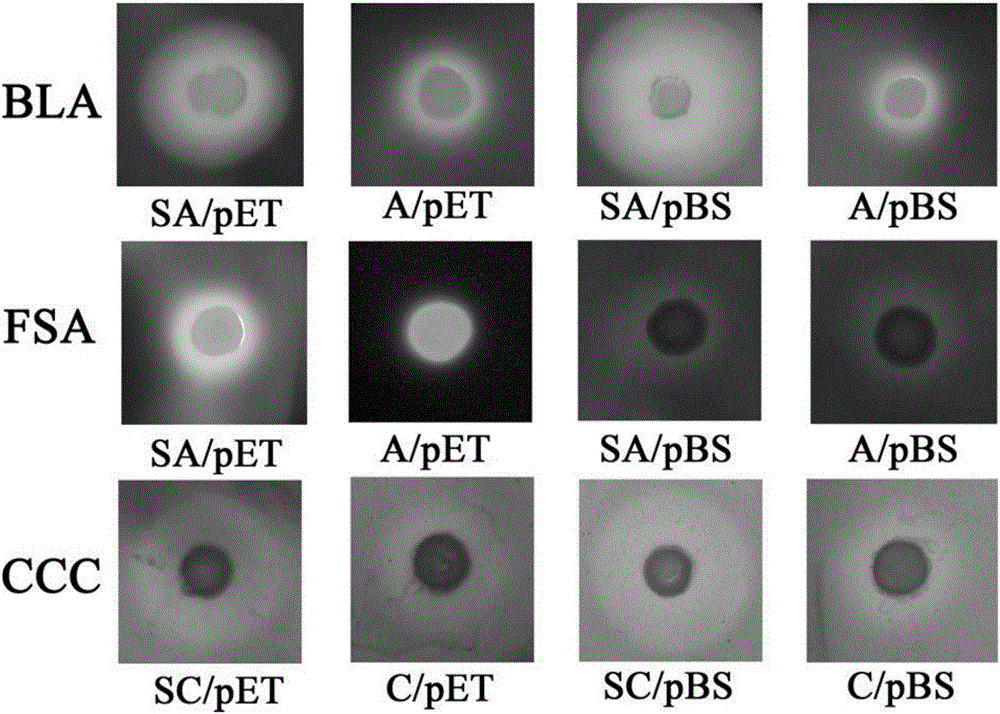
Actinomycete signal peptide for expressing intracellular protein to extracellular position and application thereof
An intracellular protein and signal peptide technology, applied in the field of genetic engineering, can solve the problems of low versatility of high-efficiency secretion and expression, no report on recombinant secretion and expression of Escherichia coli, etc. active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Construction of chemical method recombinant strain
[0029] Competent state preparation: chemical method (Ca Cb) method
[0030] Add 1% inoculum of BL21 overnight culture to 50ml LB; measure OD600nm every 15-20min, and collect the bacteria when it grows to 0.3-0.4; place the culture on ice for 10min, then pour it into a 50ml centrifuge tube , 4°C, 4500rpm, centrifuge for 10min (the 50ml centrifuge tube also needs to be pre-cooled, adjust the centrifuge to 4°C in advance); discard the supernatant, and use 30ml of pre-cooled 0.1M MgCl 2 -CaCl 2 Resuspend the cell pellet in the solution; centrifuge at 4500rpm for 10min at 4°C; discard the supernatant, and add 2ml of pre-cooled 0.1M CaCl to the 50ml initial culture medium 2 Solution (15% glycerol) to resuspend the cell pellet; aliquot 50 μl / tube or 100 μl / tube, and store at -70°C.
[0031] Ligation system: 4 μl of carrier, 5.5 μl of target fragment, T 4 DNA ligase 0.5 μl, T 4 DNA ligase Buffer 1μl; ligati...
Embodiment 2
[0032] Embodiment 2: Construction of FSA and SFSA recombinant strain
[0033] The full length of FSA amylase gene is 1359bp. Primers were designed according to the signal peptide sequence, and the signal peptide sequence was recombined with the gene sequence shown in KC441955.1 to obtain the fusion gene fragment SFSA. Among them: one round of PCR amplified the upper and lower fragments respectively, and the primers were SFSA-F1: GAGGATCCGATGAGCAGACGAGC, SFSA-R1: GCGAGCGCCGATGACAATAATAATTATAC; SFSA-F2: GTATAATTATTATTGTCATCGGCGCTCGC, SFSA-R2: GACGTCGACTTACTCTCCCGAAACCGACCATATG. The two groups of PCR products were recovered by gel cutting, the concentration of the recovered products was measured, and water was added to adjust the concentration of the upstream and downstream homology arms to be basically the same; the two rounds of PCR used the first round of PCR products as primers and templates to connect the two amplification products, The PCR products were recovered; the 3 ro...
Embodiment 3
[0037] Embodiment 3: Construction of BLA and SBLA recombinant strain
[0038] The full length of the BLA amylase gene is 1455bp. Primers were designed according to the signal peptide sequence, and the signal peptide sequence was recombined with the gene sequence shown in AAU39594 to obtain the fusion gene fragment SBLA, in which the upper and lower fragments were amplified in one round of PCR, and the primers were SBLA-F1: GAGGATCCGATGAGCAGACGAGC, SBLA- R1: CTTTAAGATTTGCCGCGGCGCTCG; SBLA-F2: GCGAGCGCCGCGGCAAATCTTAAAG, SBLA-R2: CCCAAGCTTGGGCTATCTTTGAACATAAATTG. The two groups of PCR products were recovered by gel cutting, the concentration of the recovered products was measured, and water was added to adjust the concentration of the upstream and downstream homology arms to be basically the same; the two rounds of PCR used the first round of PCR products as primers and templates to connect the two amplification products, The PCR products were recovered; the 3 rounds of PCR used...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com