Highly bioavailable oromucosal drug formulations based on cyclodextrin and sucralose
A technology of sucralose and cyclodextrin, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, and drug combinations, etc. Other effects of sexual sucralose, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
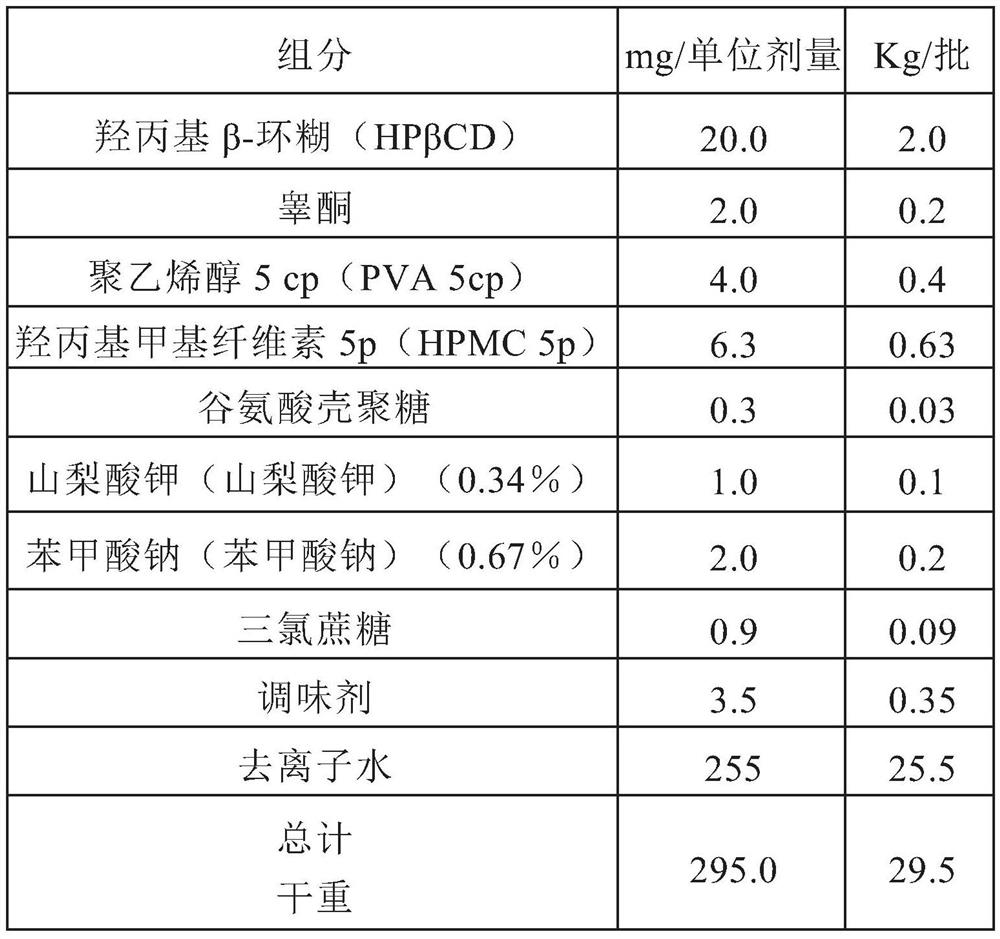
[0052] Example 1: Preparation of the ternary complex testosterone / HPBCD / sucralose (molar ratio: 1.0:2.0:0.2)
[0053] Pour 278.95 g of deionized water into the dissolver at room temperature, add 279.62 g of hydroxypropyl β-cyclodextrin and dissolve with stirring at 500 rpm until completely dissolved so that the solution appears clear with no visible residue.
[0054] 6.821 g of sucralose was added to the solution and mixed at 500 rpm for 5 minutes at room temperature until fully dissolved so that the solution appeared clear with no visible residue. 26.4 g of testosterone was added to the solution with continuous stirring until completely dissolved (solution appeared clear with no visible residue). The resulting solution was diluted with the remaining aliquot of water corresponding to 172.43 g. The mixture was stirred at room temperature at 500 rpm for 10-15 minutes to homogenize the solution.
[0055] A spray-drying process is performed to obtain the final powder (spray-dr...
example 2
[0056] Example 2: Preparation of binary complex HPβCD / testosterone (molar ratio: 2.0:1.0)
[0057] Pour 278.95g of deionized water into the dissolver at room temperature, add 279.62g of hydroxypropyl β-cyclodextrin and dissolve it by stirring at 500rpm for 5 minutes until completely dissolved so that the solution appears clear and has no visible residue things.
[0058] Under continuous stirring, 26.4 g of testosterone was added to the solution until completely dissolved (the solution appeared clear and had no visible residue. The resulting solution was diluted with the remaining aliquots of water equivalent to 172.43 g. The mixture was heated at room temperature at 500 rpm Stir for 10-15 minutes to homogenize the solution.Perform a spray drying process to obtain the final powder (spray drying mixture).
example 3
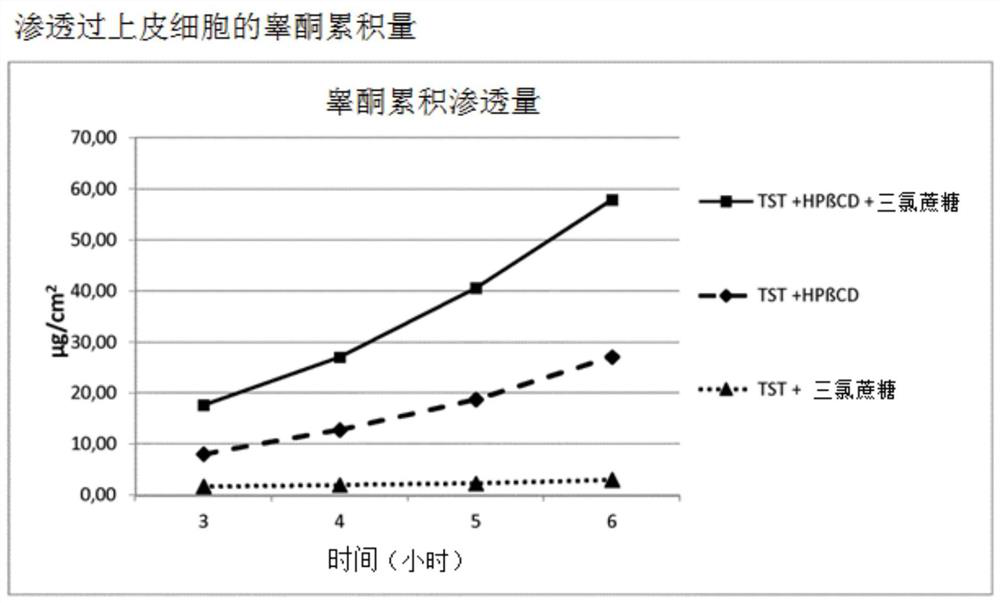
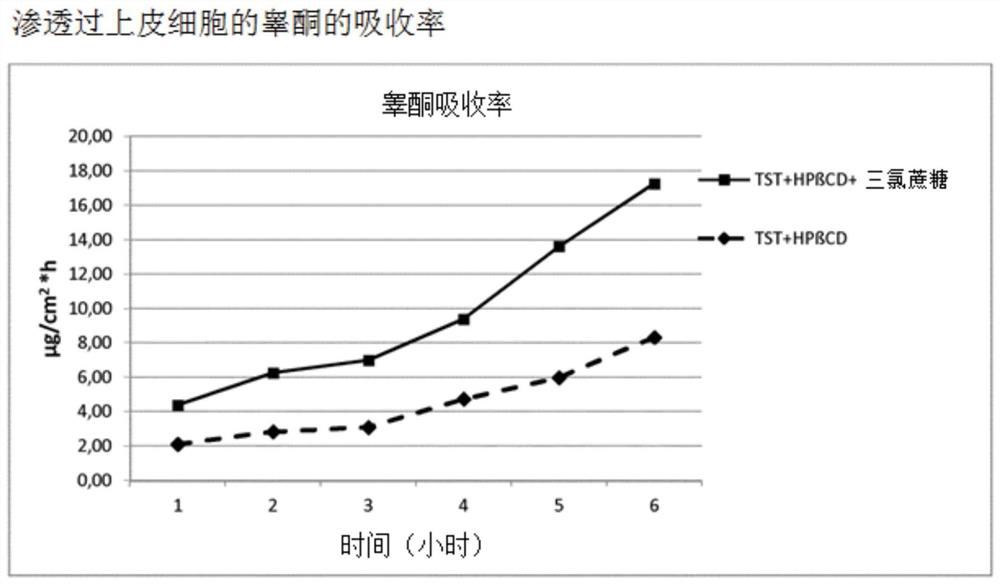
[0059] Example 3: Penetration studies through epithelial cells
[0060] The mechanism of absorption enhancement was demonstrated by testing buccal epithelial cells grown in vitro using simulated saliva at pH 6.8 (M.R.C. Marques et al., “Simulated Biological fluids with Possible application in dissolution testing”; Dissolution Technologies August 2011).
[0061] Simulated saliva at pH 6.8
[0062] Under magnetic stirring, dissolve 8.00 g of sodium chloride, 0.19 g of potassium dihydrogen phosphate and 2.38 g of disodium hydrogen phosphate in 1 L of H 2 O, after dissolution, acidify to pH 6.8 with 85% phosphoric acid.
[0063] Sample solution 1: Ternary compound of testosterone / HPβCD and spray-dried sucralose (molar ratio: 1.0:2.0:0.2)
[0064] Under slow magnetic stirring, 300 mg of the ternary complex of testosterone / HPβCD and spray-dried sucralose was dissolved in 50 mL of simulated saliva, pH 6.8. The final concentration of testosterone in solution was equal to 0.516 mg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com